Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 10
28 November 2018
CASE I: Case 1 (JPC 4006380)
Signalment: 3-year old, boxer, dog (Canis familiaris)
History: The patient initially presented to the internal medicine service at the University of Glasgow with a 1 year history of protracted colitis. An endoscopic biopsy of the colon was taken at that time. Five weeks following biopsy, the patient re-presented for acute onset of blindness. The owners reported a slight vision loss beginning roughly 1 month prior that progressed rapidly to complete blindness within the previous 24 hours.
On physical examination, there was subtle ocular hyperemia. The menace responses were absent and the PLRs very weak and sluggish. There was mild corneal edema and significant deep stromal vascularization in both eyes. There was marked aqueous flare and swollen irides. The right eye had a reddish tapetal reflex with a blood tinged and murky posterior segment with no detail discernible. The left eye had a good tapetal reflex but there was profound posterior segment disturbance with thickened folds of hyperemic and hypervascular detached retina present throughout and the impression of a cellular retinal infiltrate. A subretinal aspirate was collected and submitted to Veterinary Diagnostic Services at the University of Glasgow for cytologic analysis and routine cultures. Cytology smears were routinely stained with May-Grünwalds and Giemsa.
Gross Pathology: None given.
Laboratory results: Prototheca sp. was cultured on routine aerobic cultures. The organism was not further speciated.
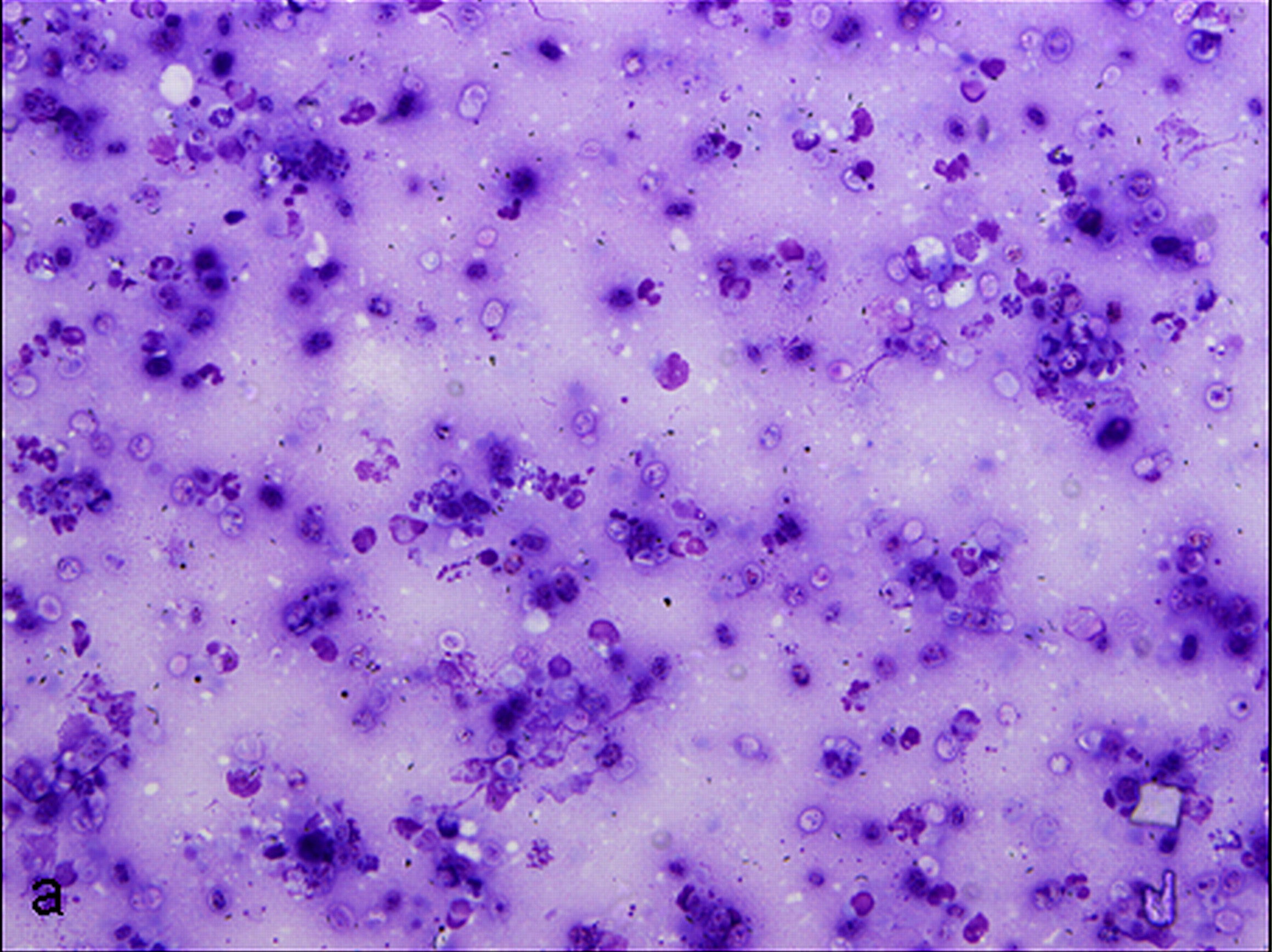
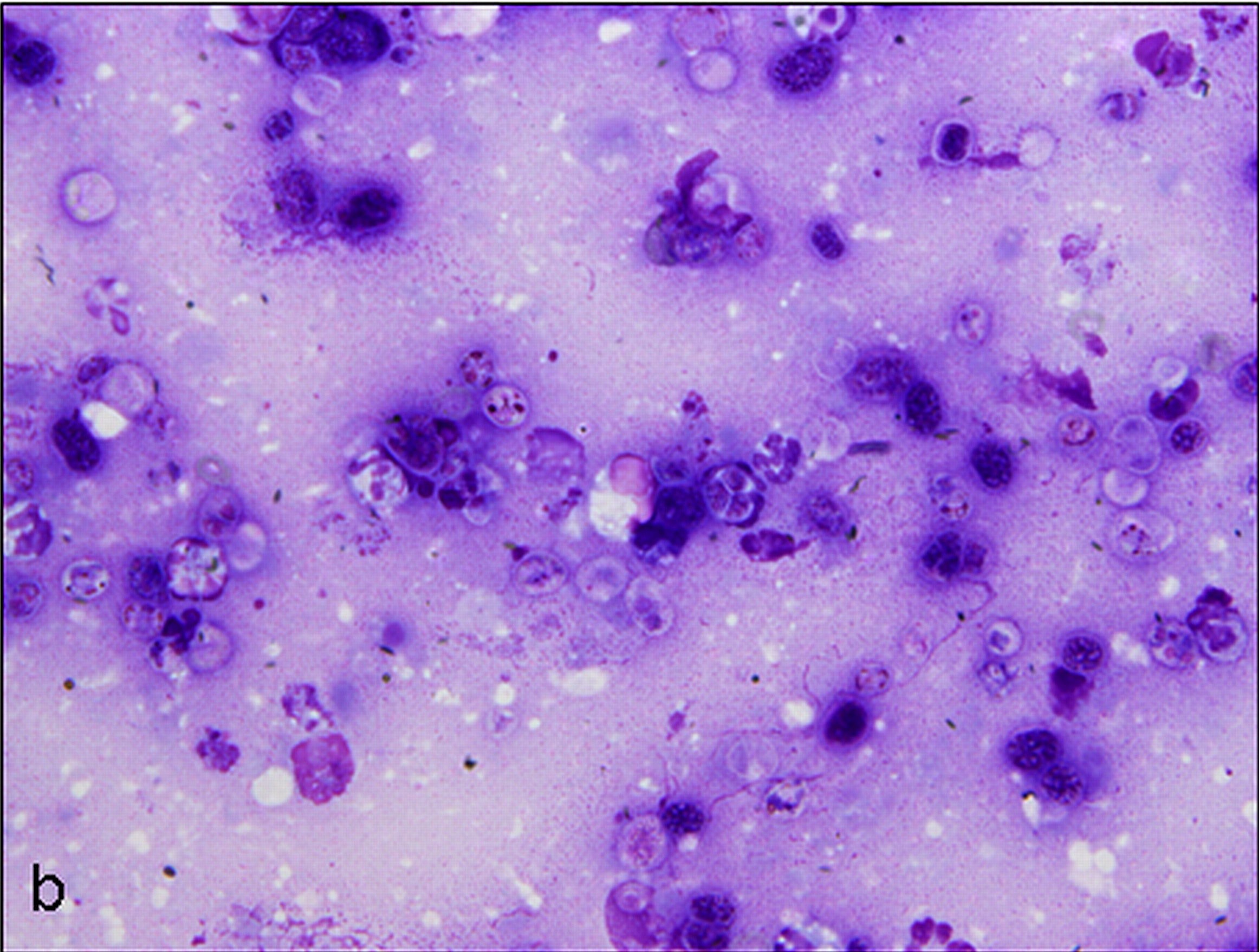
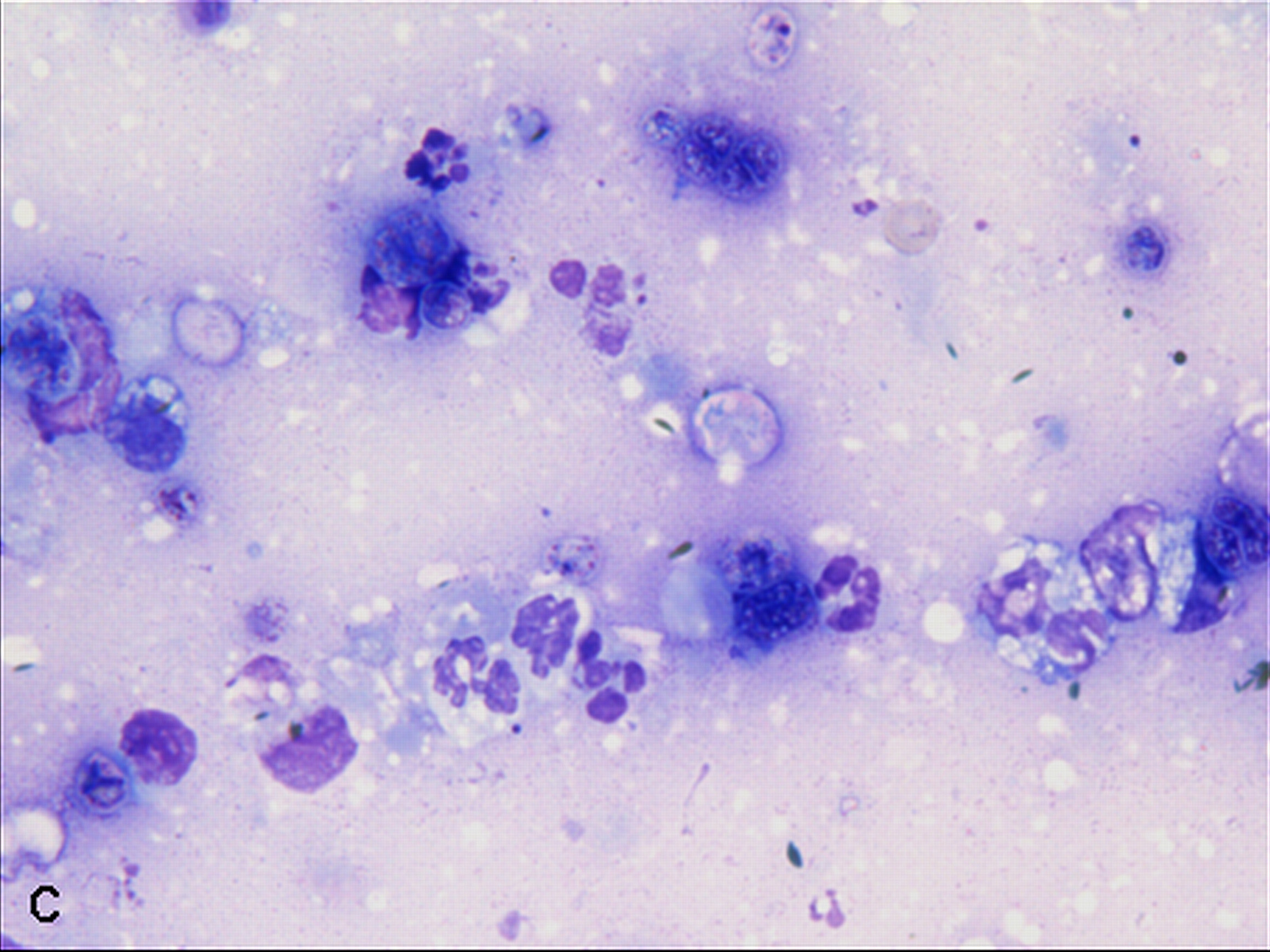
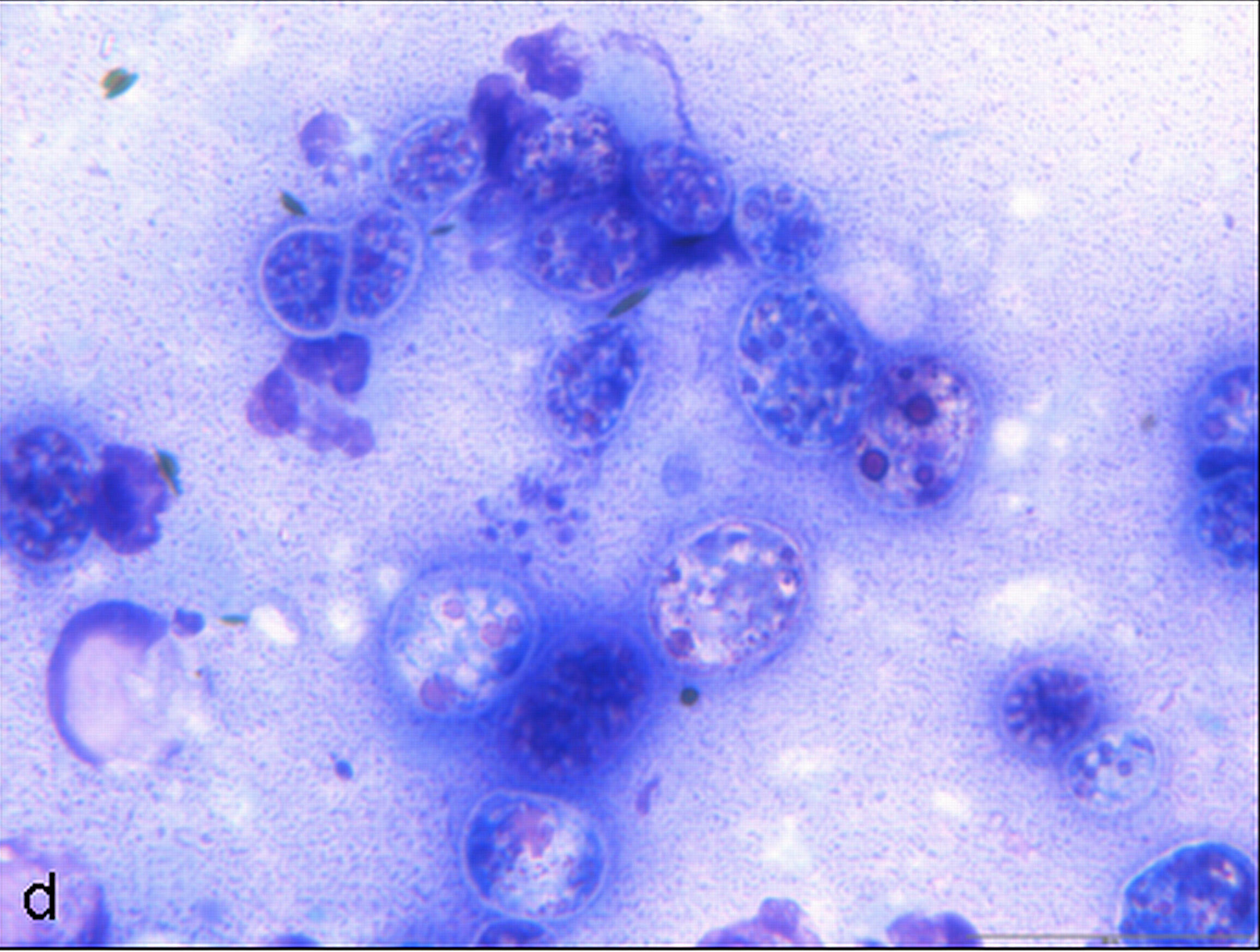
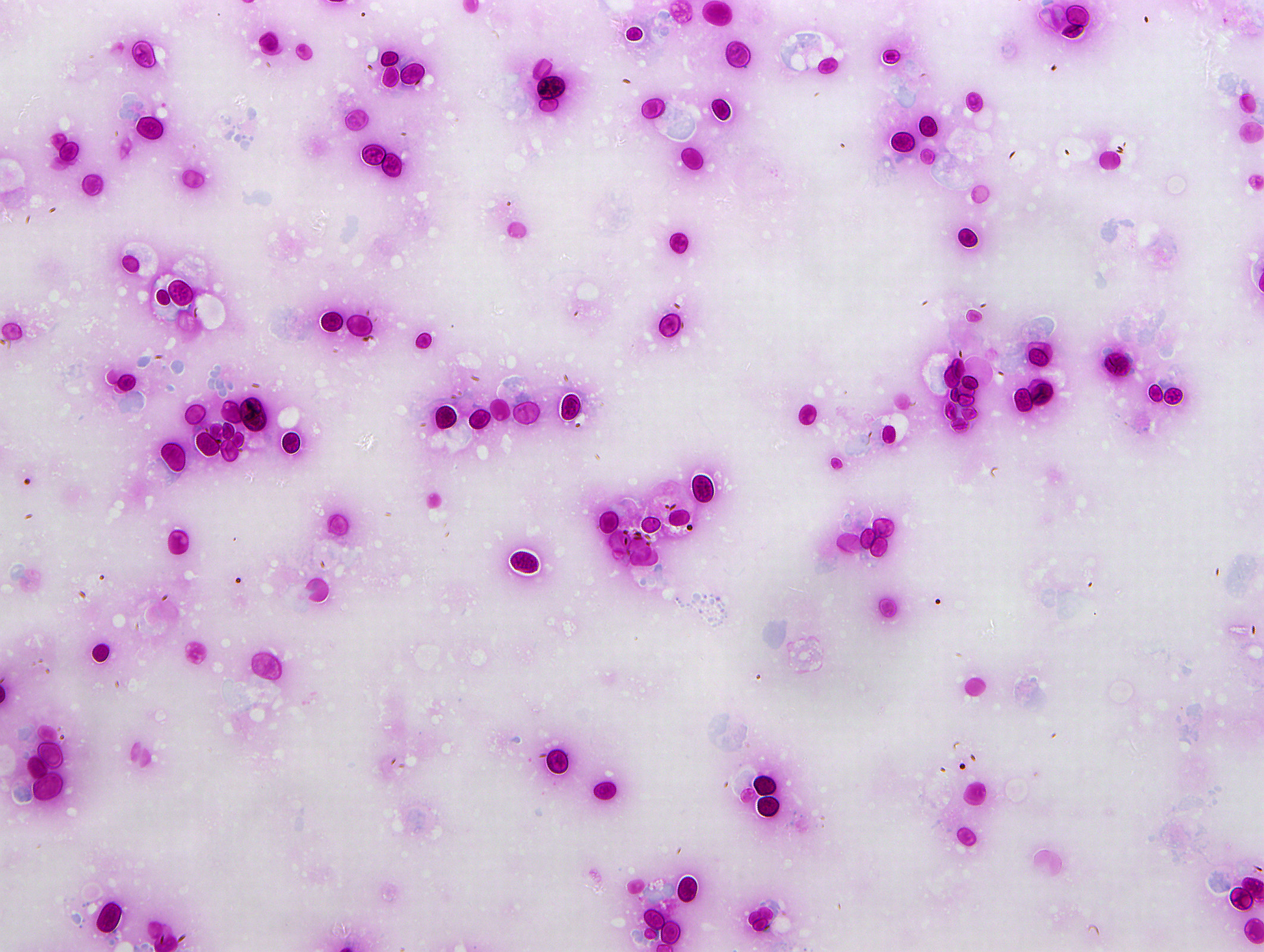
Cytologic Description:
Examined is a highly cellular smear of good quality. There is a dense, faintly basophilic proteinaceous background containing many free rod-shaped melanin granules. There are large numbers of degenerate neutrophils admixed with low numbers macrophages and occasional erythrocytes. Abundant intracellular and extracellular algal organisms are present, both intact and as ruptured empty cell casings. Algal organisms are variably sized, 5-10 μm diameter, round to oval, have a thin, clear cell wall and have deeply basophilic cytoplasm (consistent with Prototheca spp.). Early endospore replication is characterized by clusters of 3-5 endospores with deeply magenta nuclei and internal septation. Sporangia are 20 μm in diameter, have pale basophilic cytoplasm and contain up to 20, magenta, 5 μm diameter sporangiospores. Occasional organisms are seen intracellularly within the cytoplasm of degenerate cells. Organisms are Periodic Acid-Schiff (PAS) positive.
Contributor’s Morphologic Diagnoses:
Purulent inflammation with extracellular algal organisms (consistent with Prototheca spp.)
Contributor’s Comment: Prototheca spp. are unicellular, colorless, saprophytic algae that can be found within the environment, particularly with standing water and sewage effluent.6 Protothecosis is a rare disease of people and animals that can be localized, such as in the intestine or skin, or disseminated, resulting in widespread granulomatous and/or necrotizing lesions throughout the body.1 In people, infection is primarily within the skin or joints. 6 Protothecosis is a relatively uncommon cause of mastitis in cattle.1 In dogs, the intestine and the eye are the most common organs affected. 2,5,6
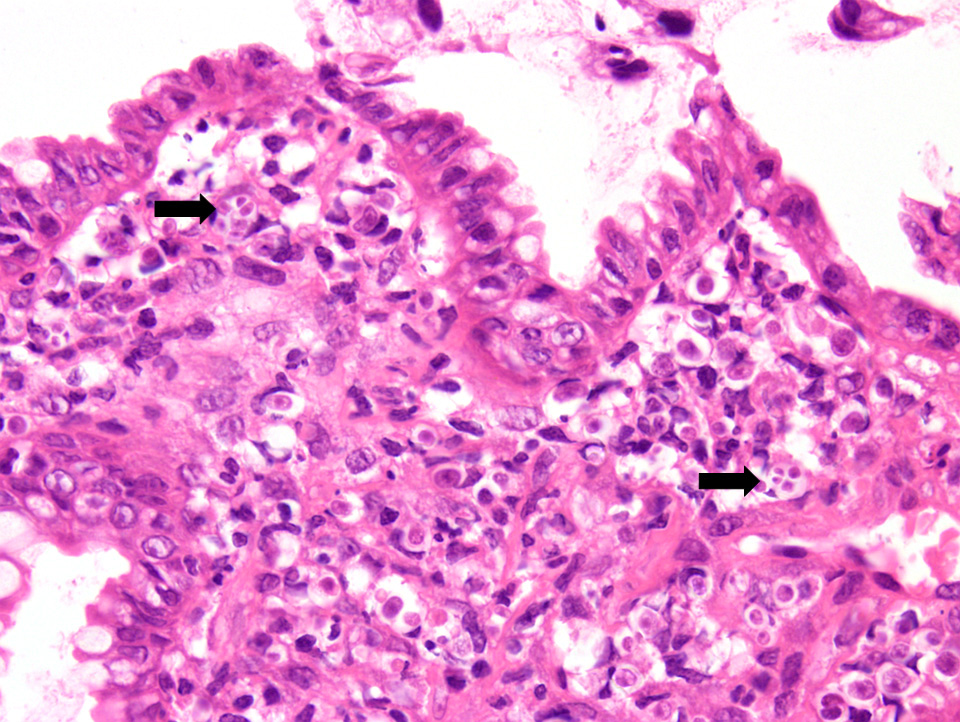
Colitis is the most common presenting complaint in dogs with protothecosis and the clinical course is often protracted. 6 Dogs can also present with acute blindness.9 The dog in this case initially presented with colitis, followed by acute blindness and had a clinical presentation typical of protothecosis. In dogs, protothecal organisms first invade and colonize the colon, with further spread through both lymphatics and blood vessels to other organ systems. In this case, biopsies from the colon in this dog revealed a marked pyogranulomatous and ulcerative colitis. Rare protothecal organisms were detected within the sections, confirming infection in both the intestine and the eye. The dog initially responded to treatment with partial resolution of the colitis, which included amphotericin B and itraconazole; however, vision never returned. The dog was eventually euthanized due to financial constraints. Disseminated infection could not be documented because post-mortem examination was declined.
Protothecal organisms can be highlighted with special stains, such as PAS and Gomori’s Methenamine-Silver, in both cytological and histopathology sections. 1 Algal organisms of the Chlorella species can be morphologically similar to Prototheca spp. Chlorella spp. can be differentiated by the often green tinge to the organisms and the presence of PAS positive cytoplasmic granules that are negative following amylase digestion.1 The two main species of Prototheca known to cause infection in dogs are P. zopfii and P. wickerhamii. Though these species of Prototheca differ slightly morphologically (size and number of sporangiospores), PCR is required for definitive differentiation.
Differentials for suppurative or pyogranulomatous inflammation in ocular fluid include both bacterial and fungal infections. 9 Rarely, protozoa, such as Toxoplasma gondii, can result in endophthalmitis.9 Any case of bacteremia has the potential to develop bacterial endophthalmitis and the list of potential agents is extremely long.9 Fungal endophthalmitis is also typically secondary to a mycotic septicemia. Fungal organisms most commonly associated with eye infections include Cryptococcus neoformans, Blastomyces dermatitidis, Histoplasma capsulatum (particularly in cats), and, less likely, Coccidioides immitis.9 Both bacterial and fungal infections of the eye are often differentiated based on morphology of the organism, including size and presence of budding, and/or culture. Cultures can be obtained from endoscopy samples, ocular fluid, and/or urine of living patients, depending on the organ systems involved clinically.
Contributing Institution:
School of Veterinary Medicine
University of Glasgow
Garscube Estate
Bearsden, Glasgow
G611QH, United Kingdom
http://www.gla.ac.uk/schools/vet/
JPC Diagnosis: Fine needle subretinal aspirate: Pyogranulomatous inflammation with numerous endosporulating alga.
JPC Comment:
Four species of Prototheca exist: P. zopfii, P. wickerhamii (the two pathogenic forms), P. stagnora, and a proposed form, P. salmonis. They are found in abundance in nature in animal waste, sewage, soil, food, and flowing or standing water and have a worldwide distribution. Additional ribosomal sequencing further divided P. zopfii into biotypes, with P. zopfii biotype one being present in bovine liquid manure, biotype 2 present in many cases of bovine mastitis, and biotype 3 isolated from swine manure (and recently renamed P. blaschkeae.)4 A novel Prototheca (P. cutis sp. nov) has been tentatively identified based on ribosomal RNA sequencing of a single human isolate.4
Despite its ubiquity, animal infections are rare, with dogs, cats and cattle predominantly reported in the literature. Cats are most commonly infected cutaneously with large firm nodules on the limbs and feet, and P. wickerhamii is most commonly isolated.7 In cattle, P. zopfii is an uncommon cause of pyogranulomatous lesions of the bovine mastitis and associated lymph nodes.4 In goats, P. wickerhamii causes ulcerative nodules of the mucocutaneous junction of the nasal vestibule, nostrils, and subcutis of the face and head.4
Human infections are uncommon and usually manifest as erythematous vesicobullous rashes which may occasionally be crusting and purulent. Risk factors include immune suppression from HIV, prolonged steroid use, diabetes mellitus, and underlying malignancy.2 The frequency of cases increases with age. Most human cases are the result of infection with Prototheca wickerhamii. Disseminated infections, although uncommon and usually seen only in patients with severe immunodeficiency, and demonstrate a mortality rate of 60% (in a limited number of reported cases.2
A related non-photosynthetic of algae, Helicosporidium, has recently been identified as a specialized parasite from a variety of dipteran, coleopteran, and lepidopteran insects, primarily infecting larvae.8
References:
- Brown CC, Baker DC, Barker IK. Alimentary System. In: Maxie MG ed. Pathology of Domestic Animals. 5th Edinburgh, UK: Saunders Elsevier; 2007: 231-232.
- Hillsheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med 2011; 135: 941-946.
- Hollingsworth SR: Canine protothecosis. Vet Clin North Am Small Animal Pract. 2000; 30:1091-1101.
- Pal M, Abraha A, Rahman MT, Dave P. Protothecosis: an emerging algal disease of humans and animals. Int J Life Sc Bt Pharm Res
- Pier AC, Cabañes FJ, Chermette R, Ferreiro L, Guillot J, Jensen HE, Santurio JM. Prominent animal mycoses from various regions of the world. Med Mycol. 2000; 38 Suppl 1:47-58.
- Stenner VJ, Mackay B, King T, Barrs VR, Irwin P, Abraham L, Swift N, Langer N, Bernays M, Hampson E, Martin P, Krockenberger MB, Bosward K, Latter M, Malik R. Protothecosis in 17 Australian dogs and a review of the canine literature. Med Mycol. 2007; May;45(3):249-66.
- Strunck E, Billups L, Avgeris S. Canine protothecosis. Compendium 2004; 96-102
- Tartar, A. The non-photosynthetic algase Helicosporidium : emergence of a novel group of insect pathogens. Insects 2013; 4:375-391.
- Wilcock BP. Eye and Ear. In: Maxie MG ed. Pathology of Domestic Animals. 5th ed. Edinburgh, UK: Saunders Elsevier; 2007: 231-232.