Signalment:
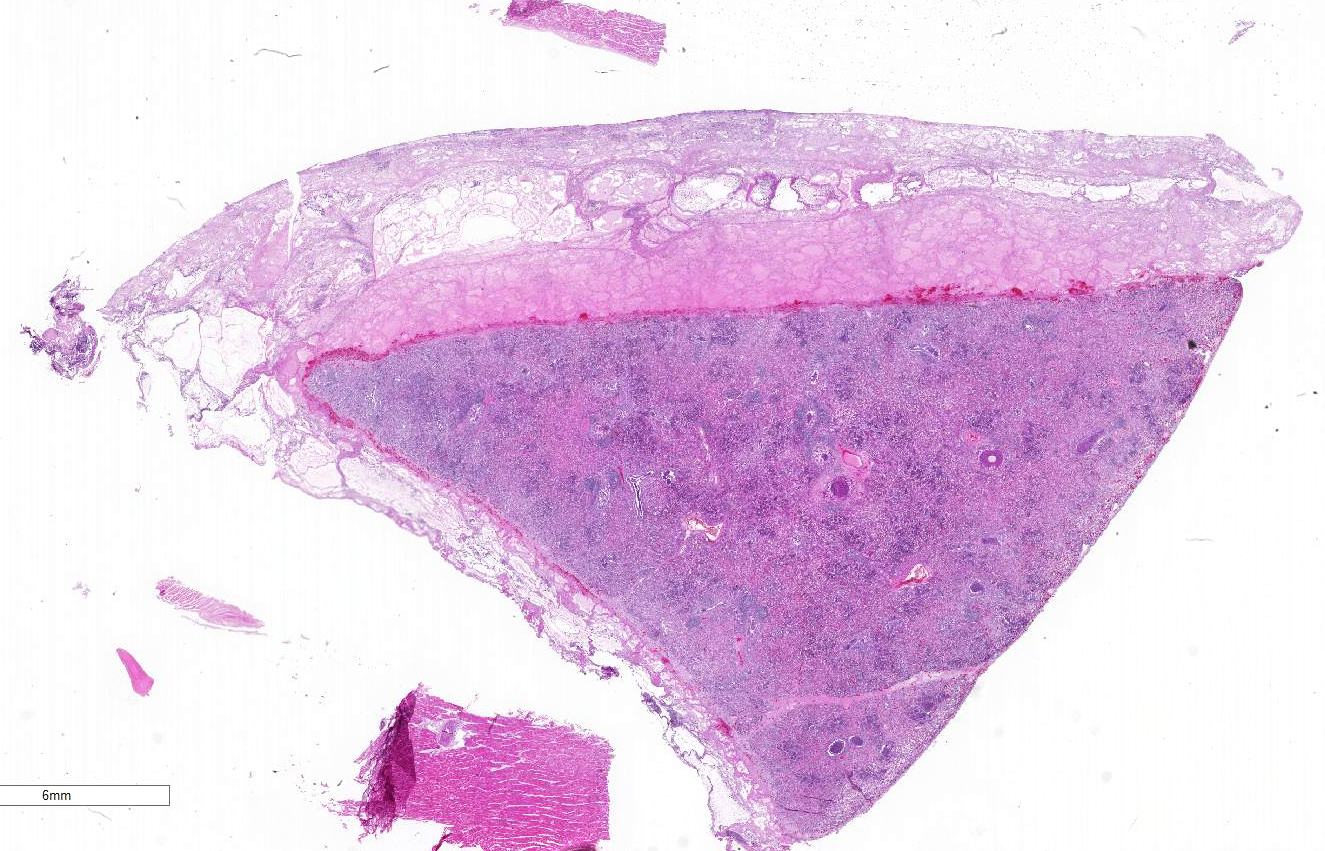
Gross Description:
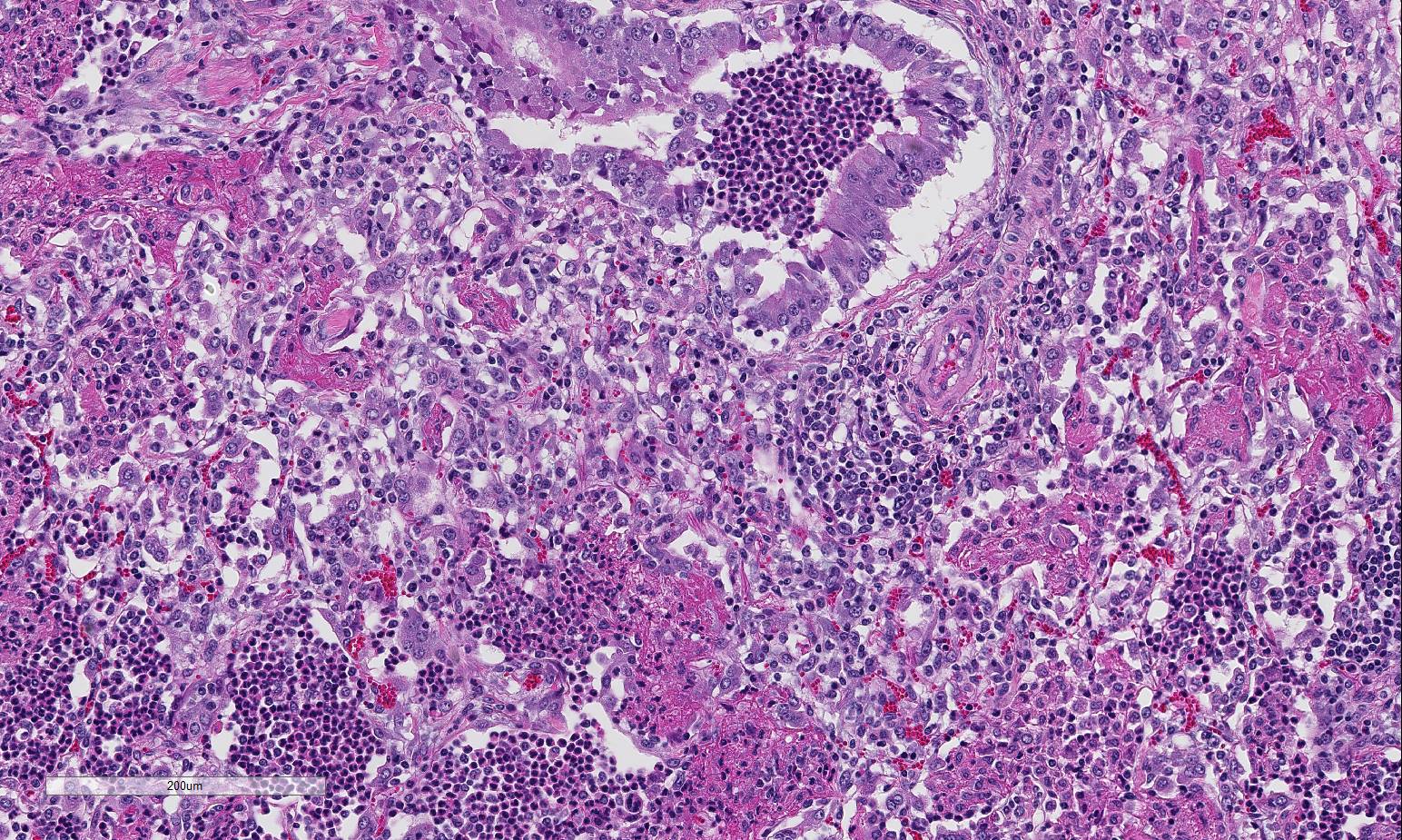
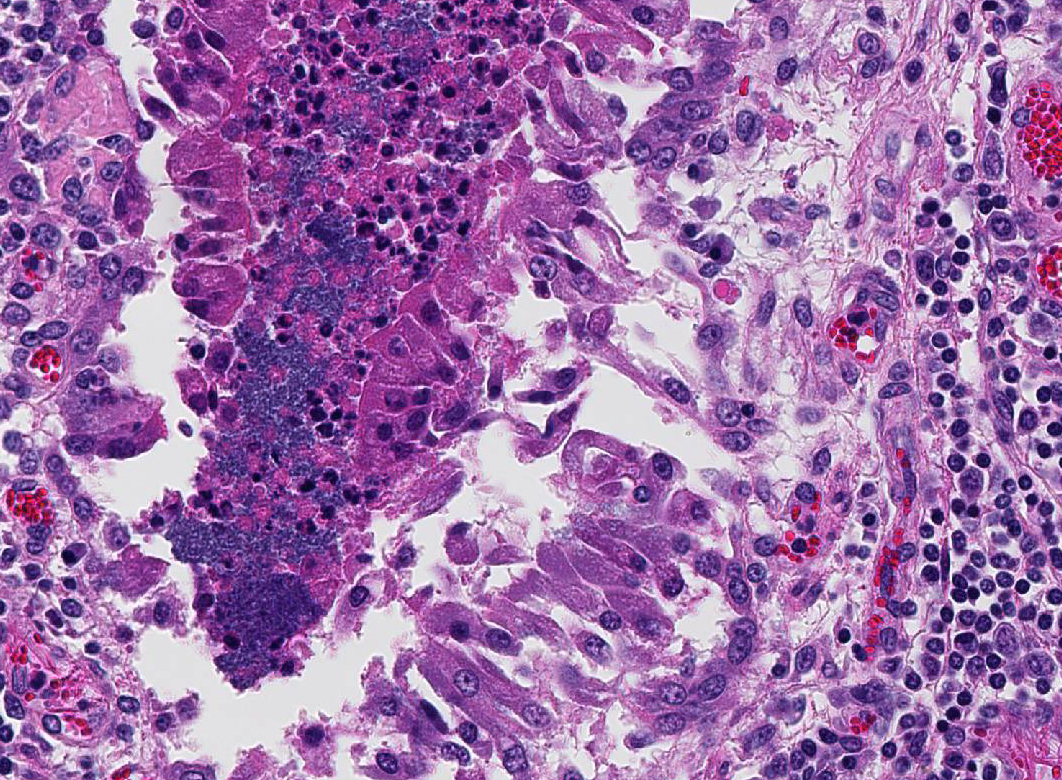
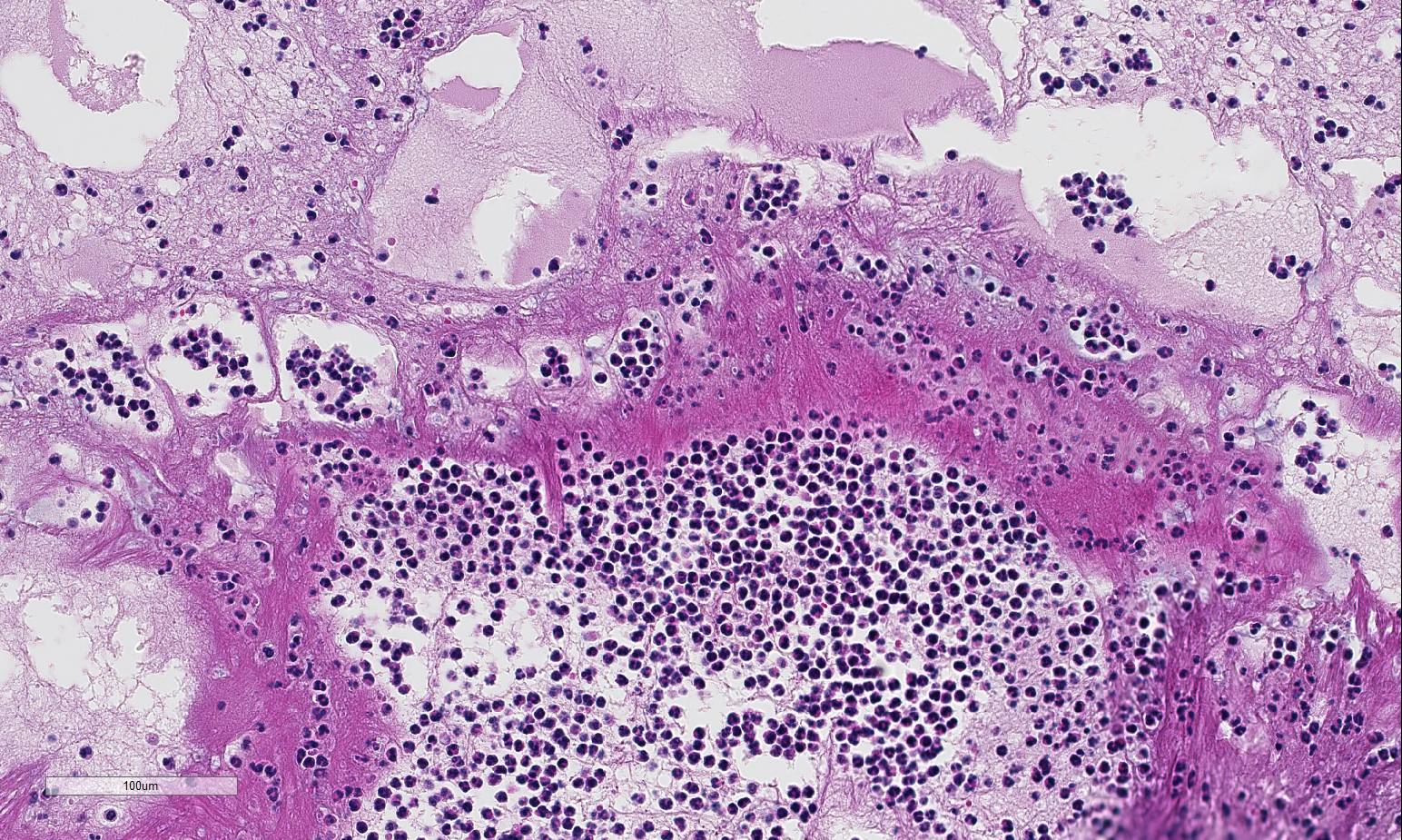
Histopathologic Description:
Morphologic Diagnosis:
Lung: Severe necrotizing and fibri-nosuppurative bronchopneumonia, with intralesional bacterial bacilli; Severe, fibrinous pleuritis; Moderate, lymphocytic interstitial pneumonia (caudodorsal lung, not provided).
Lab Results:
Bacterial culture and sensitivity (lung):
(HE, 5X)">Moderate Mannheimia haemolytica; Moderate alpha hemolytic Streptococcus; Few Trueperella pyogenes
Qualitative fecal analysis:
Moderate # coccidial oocysts; Many trichostrongyle type eggs; Few Trichuris eggs
Condition:
Contributor Comment:
Mannheimia haemolytica (formerly Pasteurella haemolytica, biotype A), a gram-negative coccobacillus, is a common cause of fibrinous and necrotizing pn-eumonia and pleuropneumonia in cattle, sheep, and goats. Although pneumonic ma-nnheimiosis has been most extensively investigated in bovines, pathologic lesions in sheep are essentially similar to those described for cattle, with few differences. In sheep, the disease may be acute or chronic. In the acute form, there may be a hem-orrhagic or fibrinonecrotic lobar pneumonia and fibrinous pleuritis, whereas in the chronic form, a fibrinopurulent bron-chopneumonia with secondary abscessation and fibrinous adhesions to the thoracic wall are common. Additionally, serous to serofibrinous fluid may also be seen in the pericardial sac and pleural and peritoneal cavities. 5
Mannheimia haemolytica is an opportunistic pathogen; it is a normal inhabitant of the nasopharynx and tonsils of cattle and sheep. 3,7 Although the exact mechanisms are not known, stress or concurrent viral infections may alter the local innate and adaptive immune responses, contributing to the development of disease. The organism also possesses several virulence factors, in-cluding leukotoxin (LKT), lip-opolysaccharide (LPS), adhesins, capsule, outer membrane proteins, and various pro-teases which allow promote colonization of the lung and evasion of the host immune response. 3,5,7
The roles of LKT and LPS in the pathogenesis of pulmonary mannheimiosis are most well known in bovines with similar effects in small ruminants. LKT is a 102- to 105-kDa protein that is produced by all serotypes during the logarithmic phase of bacterial growth. 3,5,7 This pore-forming cytolysin is a member of the RTX (repeats in ToXin) family of toxins. These toxins are genetically related, sharing a highly conserved motif consisting of a series of glycine-aspartic acid nonapeptide repeats in the carboxy terminal third of the LKT protein molecule. 7 This motif serves two critical functions; it is involved in calcium binding which induces leukocyte toxicity and contains a recognition site required for transport of LKT across biological mem-branes in bacteria. 7 Although members of the RTX family of toxins are genetically similar, they differ in the target cell specificity. Other members of the RTX family and their toxins include: Actinobacillus actinomycetemcomitans (LtxA), Actinobacillus pleuropneumoniae cytotoxins (ApxI, ApxII, ApxIII, ApxIV), Escherichia coli alpha hemolysin, Actinobacillus suis subs haemolyticus toxin (Aqx), Fusobacterium necrophorum LKT, Bibersteinia trehalosi, and Bordetella pertussis hemolysin. 7
Pneumonia due to Mannheimia haemolytica occurs only in ruminants, in part due to LKT-induced effects which are specific for ruminant macrophages, lymphocytes, neutrophils, and platelets.3,5,7 The response to LKT is species-specific and dose-dependent. Species specificity is due to the selective interaction of LKT with ?2 integrin LFA-1 (lymphocyte function-associated antigen 1; CD11a/CD18 on target host cells). 7 At low levels, LKT activates neutrophils and macrophages to stimulate respiratory burst and degranulation, release of proinflammatory cytokines TNF?, IL-1, and IL-8 and histamine from mast cells, and inhibit lymphoid proliferation.7 At higher concentrations, leukocytes undergo ap-optosis, whereas at highest concentrations, LKT causes transmembrane pore formation, cell swelling, and oncotic cell death.3,5,7 Pulmonary damage occurs subsequent to the leakage of oxygen free radicals, superoxide anions, lysosomal enzymes, and arachidonic acid metabolites into pulmonary parenchyma.7
Pulmonary lesions also occur due to LPS, a molecule composed of polysaccharide side chain (O antigen), lipid A, and inner and outer cores of oligosaccharides. Lipid A is responsible for eliciting endotoxic effects, such as fever and hypotensive shock. 1 It is a potent vasodilator, is directly toxic to pulmonary endothelium, and can recruit neutrophils.3,5 LPS stimulates alveolar macrophages to produce proinflammatory cytokines, reactive oxygen and nitrogen intermediates, and other mediators that participate in the inflammatory process, including IL-1?, IL_8, leukotriene 4, prostaglandin E2, and TNF? from leukocytes.7 An influx of neutrophils occurs secondary to these proinflammatory cy-tokines and chemotactic mediators. LPS also enhances the effects of LKT. 7
Although the sections of caudodorsal lung were not submitted, the lesions are worth mentioning as they were most suggestive of ovine lentiviral pneumonia, or maedi-visna (ovine progressive pneumonia). Lentiviruses (Retroviridae) cause slowly progressive inflammation in a variety of tissues, with the lung, CNS, mammary gland, and joint most commonly affected.1 Syndromes may occur independently or concurrently in any combination. The primary pathologic ch-ange is infiltration of lymphocytes into the affected tissues, with occasional formation of lymphoid follicles similar to those seen in lymph nodes.1 The respiratory form in sheep, maedi (“dyspnea in Icelandic) is the most common form in sheep usually greater than 3 years of age. Encephalitis, or visna (“fading away” in Icelandic), may present as ataxia, trembling, and significant weight loss. Mastitis and arthritis also occur, but with little frequency. Small ruminant lentiviruses are primarily transmitted through colostrum or milk, although in-halation of respiratory secretions can occur.1 In this case, despite the history of ne-urologic signs, lesions associated with lentiviral infection were not detected in the brain. A commercially available test to confirm this infection is not routinely available, however, given the clinical history and gross and histopathological findings in the lung, lentiviral infection remains a likely differential in this case.
JPC Diagnosis:
1. Lung: Bronchopneumonia, fib-rinosuppurative, chronic-active, diffuse, severe with fibrinous pleuritis, multinucleate giant cells, and colonies of bacilli.
2. Lung: Interstitial pneumonia, ly-mphohistiocytic, multifocal, mild.
3. Heart: Essentially normal tissue.
Conference Comment:
)and P. multocida may be indistinguishable, as all include a cranioventral, lobular distribution with or without the presence of fibrinous pleuritis, although well-demarcated foci of necrosis will distinguish both M. haemolytica and H. somni. Albeit characteristically attributed to M. haemolytica infection, leukocyte necrosis may also be seen in cases of H. somni infection. The presence of suppurative phlebitis with fibrinoid necrosis is more common in cases of H. somni infection.2 M. hemolytica is also a common cause of mastitis in sheep, which can be a problem in both dairy and non-dairy flocks. In addition to M. hemolytica, M. glucosida may also cause pneumonia and mastitis in sheep. Horizontal transmission is common, with nursing being suggested as the major method of transmission, where the organism is passed to the mammary gland from the nasopharynx of the lamb.6
Besides the obvious fibrinosuppurative bronchopneumonia in the submitted se-ctions, there was extensive discussion conference participants also identified a mild interstitial pneumonia with infiltration and expansion of alveolarsepta. The mild interstitial component along with occasional albeit prominent cuffing of vessels and small airways by mononuclear inflammatory cells (with a majority of lymphocytes) generated discussion of a concomitant lentiviral infection Lentiviral pneumonia, as discussed above, was postulated to be present in the caudal-dorsal sections of lung which were not submitted. Although not conclusive, viral infection may have, or perhaps likely, resulted in a secondary bronchopneumonia in this animal. Ovine progressive pneumonia (maedi-visna) may cause severe interstitial pneumonia with BALT hyperplasia, thickened alveolar septa and peribronchial lymphocytic infiltrates. Al-veolar fibrosis and smooth muscle hyperplasia, the latter of which was described in this case, may also be seen.4 The presence of multinucleated giant cells within alveoli was perplexing to some conference participants. However, ma-crophages and multinucleated histiocytes may be seen in more chronic cases of bacterial bronchopneumonia, particularly in areas of abundant fibrin exudation as they help facilitate removal of fibrin.2 Many conference participants supposed an etiologic diagnosis of Pasteurella multocida in this case due to absence of characteristic leukocyte necrosis with the linear streaming pattern, termed “oat cells,” typically seen in Mannheimia infection, as well as the lack of significant epithelial necrosis in airways (described as a feature of P. multocida infections.)
References:
1. Blacklaws BA. Small ruminant lentiviruses: Immopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp Immun, Micro, and Inf Dis. 2002; 35: 259-269.
2. Caswell JL, Williams KJ. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. St. Louis, MO: Elsevier; 2016:544-547.
3. Jeyaseelan S, Sreevatsan S, Maheswaran SK. Role of Mannheimia haemolytica leukotoxin in the pathogenesis of bovine pneumonic pasteurellosis. Anim Health Res Rev. 2002; 3(2): 69-82.
4. Lopez A. Respiratory system, mediastinum, and pleurae. In: Zachary JF, McGavin MD. eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis; Mosby Elsevier; 2012:516-517.
5. Mohamed RA, Abdelsalam EB. A Review of Pneumonic Pasteurellosis (Respiratory Mannheimiosis) with Emphasis on Pathogenesis, Virulence Mechanisms, and Predisposing Factors. Bulg J of Vet Med. 2008: 11(3): 139-160.
6. Omaleki L, Browning GF, Allen JL, Barber SR. Molecular epidemiology of Mannheimia haemolytica and Mannheimia glucosidal associated with ovine mastitis. J Diag Invest. 2012; 24(4):730-734.
7. Singh K, Ritchey JW, Confer AW. Manneheimia haemolytica: Bacterial-Host Interactions in Bovine Pneumonia. Vet Path. 2011; 48(2): 338-348.