Signalment:
11-year-old male rhesus macaque, (
Macaca mulatta).Presented for chronic weight loss (9% loss in previous 10 weeks) and past history of vaccination with commercial Fluvax-� and trauma to the hand that required surgical repair.
Gross Description:
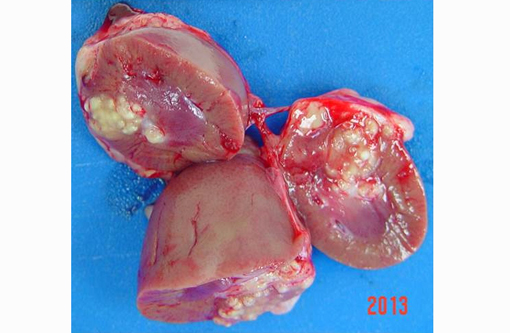
Thin male monkey presented with a bulging multinodular mass on the anterior pole of the right kidney measuring 1x2.3 cm. Suppurative exudate was apparent on cut section.
Histopathologic Description:
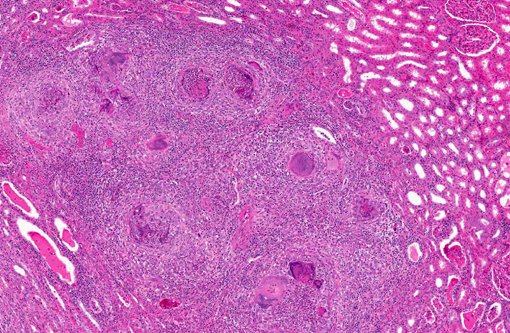
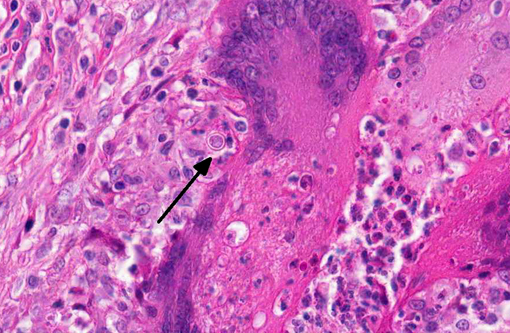
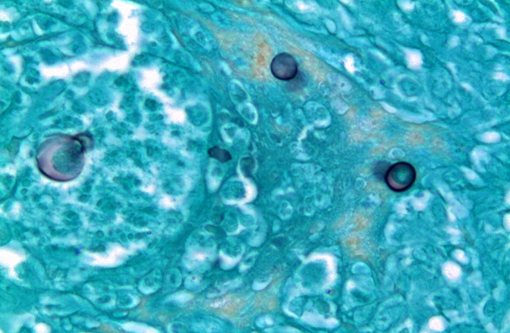
Kidney: The kidney contains multiple variably sized sometimes confluent pyogranulomas that compress and ablate parenchyma from the medulla to the capsule. Pyogranulomas have thin peripheral concentric fibrous rings infiltrated with lymphocytes and plasma cells that surround sheets of epithelioid macrophages, numerous multinucleated giant cells, and a central core filled with large numbers of neutrophils and eosinophils. Within many of the granulomas and often associated with giant cells are spherical yeast-like cells measuring 10-20 microns in diameter with double-contoured walls and a smaller dense granular central nucleoid. Rare budding forms are observed. GMS stain highlights the outer wall. Gram stain demonstrates gram positive cocci within phagocytic cells. Proximal tubules have swollen epithelium with granular sometimes vacuolated cytoplasm, while scattered groups of ectatic distal tubules contain hyaline protein and cellular casts.
Morphologic Diagnosis:
Rhesus macaque, kidney: Pyogranulomatous inflammation with intralesional
Blastomyces dermatitis and
Staphylococcus sp. and with tubular degeneration and cast formation.
Lab Results:
Previous viral testing indicated positive tests for CMV, LCV, RRV, and SV40. All previous TB skin tests were negative. CBC and clinical chemistry were unremarkable. Routine culture of the mass identified a mixture, in order of most abundant, of hemolytic, coagulase negative
staphylococcus, Streptococcus fecalis, gamma and alpha hemolytic
streptococcus, and
Corynebacterium sp. Stains of smears for AFB were negative.
Condition:
Blastomyces dermatitidis
Contributor Comment:
Blastomycosis is a disseminated or localized mycotic infection of primarily man and dogs in the Ohio and Mississippi River basins of North America and in Africa, and is caused by
Blastomyces dermatitis.(1,2) It is a dimorphic fungus found in soil in mycelial form but transforms into a pathogenic yeast at body temperature and is transmitted by inhalation or inoculation.(3) Common sites of infection are lung, skin, bone, male urogenital system, particularly prostate and testis(4) with less frequent distribution to liver, spleen, and lymph nodes.(5) It is rarely reported in monkeys; the first report in monkeys described an animal that originated from our colony.(6) We subsequently have seen several additional cases, which like the first report, developed lesions several years after leaving our breeding facility and contact with soil (Didier, unpublished). Serology(9) and PCR(11) may aid in the clinical diagnosis and recent work has associated clinical phenotypes of human infection with genetic variants of
B. dermatitidis.(12)
JPC Diagnosis:
Kidney: Pyogranulomas, multiple and coalescing, with renal capsular fibrosis, perirenal granulomatous steatitis and numerous yeasts, etiology consistent with
Blastomyces dermatitidis.
Conference Comment:
Participants summarized the key morphologic features used to differentiate
Blastomyces dermatitis from other pathogenic dimorphic fungi of veterinary significance, including
Cryptococcus neoformans, Histoplasma capsulatum, Sporothrix schenckii, Coccidioides immitis and
posadasii and
Penicillium marnefii. B. dermatitidis appears in animal tissues, both extracellularly and intracellularly within macrophages and neutrophils, as an 8-20 μm round, yeast, with a double contoured, often refractile cell wall and broad-based budding. It stains with GMS, Gridleys and PAS.Â
C. neoformans, which is also found in tissue in the yeast form, ranges from 2-20 μm and can be differentiated by its thick carminophilic capsule and narrow-based budding. Rare forms of
C. neoformans lack a capsule, making definitive identification of these yeasts a challenge.Â
H. capsulatum is smaller (2-6 μm) and typically intrahistiocytic, usually with many organisms in each macrophage and has narrow-based budding and a shrinkage-artifact halo on H&E sections; this yeast has no true capsule.Â
S. schenckii appears in tissues as a 2-6 μm, pleomorphic, often cigar-shaped intracellular and extracellular yeast. It stains well with both PAS and GMS; however, yeasts are often difficult to see on H&E-stained sections in most species other than cats, in which they are usually readily visible.Â
C. immitis and
posadasii reproduce by endosporulation and have a characteristic tissue section appearance as 20-200 μm spherules, with a double-contoured wall and numerous 2-5 μm endospores.(2) As emphasized by one participant,
Coccidioides sp. is another fungal organism occasionally encountered in research monkeys.(7)
Penicillium marnefii, which is emerging as an important pathogen of immunosuppressed humans in Southeast Asia, has also been identified in animals.Â
P. marnefii is found both intracellularly and extracellularly; yeasts are 2-3 μm and multiply by binary fission, so elongated cells up to 13 μm are occasionally present.(10)
The most important virulence factor associated with
B. dermatitidis is the adhesin BAD1. BAD1 has multiple functions and is secreted exclusively by the yeast form of Blastomyces. It mediates adhesion to host macrophages via calcium-dependent attachment to chitin in the yeast cell wall and binding to CR3 and CD14 on phagocytic cells. It also acts as an immunomodulator by suppressing the generation of TNF-α, a pro-inflammatory cytokine important in defense against fungal infections.(8)
One interesting aspect of this case is that this monkey developed clinical signs of blastomycosis several years after suspected exposure. Since the infective (mycelial) form of
B. dermatitidis is generally found in soil, and this animal was housed entirely indoors, it is presumed that he was initially infected while housed outdoors at the breeding facility, and then harbored the organism for several years before developing clinical disease.(6) As pointed out by the contributor, there have been several similar reports, which suggest the potential for long periods of subclinical infection with
B. dermatitidis before the manifestation of clinical disease.Â
References:
1. Bradsher RW. Histoplasmosis and blastomycosis. Clin Inf Dis. 1996;22(Suppl 2):S102-11.
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed., vol 2. St. Louis, MO: Elsevier Limited; 2007:523-653.
3. Khansari N, Segre D, Segre M. Diagnosis of histoplasmosis and blastomycosis by an antiglobulin hemagglutination test. Am J Vet Res. 1982;43(12):2279-2283.
4. Legendre AM. Canine blastomycosis: a review of 47 clinical cases. J Am Vet Assoc. 1981;178:1163-1168.
5. Motswaledi HM, Monyemangene FM, Maloba BR, et al. Blastomycosis: a case report and review of the literature. Inter J Derm. 2012;51:1090-1093.
6. Meece JK, Anderson JL, Gruszka S, et al. Variation in clinical phenotype of human infection among genetic groups of Blastomyces dermatitidis. J Inf Dis. 2013;207:814-822.
7. Mense MG, Batey KL, Estep S, Armstrong K, Fleurie G, Suttie AW. Disseminated Coccidiomycosis in a Cynomolgus Monkey (Macaca fasicularis). J Primatol. 2013;2(2):1-3.
8. Rappleye CA, Goldman WE. Defining virulence genes in the dimorphic fungi. Annu Rev Microbiol. 2006;60:281-303.
9. Sidamonidze K, Peck MK, Perez M, et al. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J Clin Microbio. 2012;50(5):1783-1786.
10. Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95-110.
11. Wilson RW, van Dreumel AA, Henry JNR. Urogenital and ocular lesions in canine blastomycosis. Vet Pathol. 1973;10:1-11.
12. Wilkinson LM, Wallace JM, Cline JM. Disseminated blastomycosis in a rhesus monkey (Macaca mulatta). Vet Pathol. 1999;36:460-462.