Wednesday Slide Conference, Conference 8, Case 3
Signalment:
Horse (Equus caballus), Iceland pony, adult, male neutered.
History:
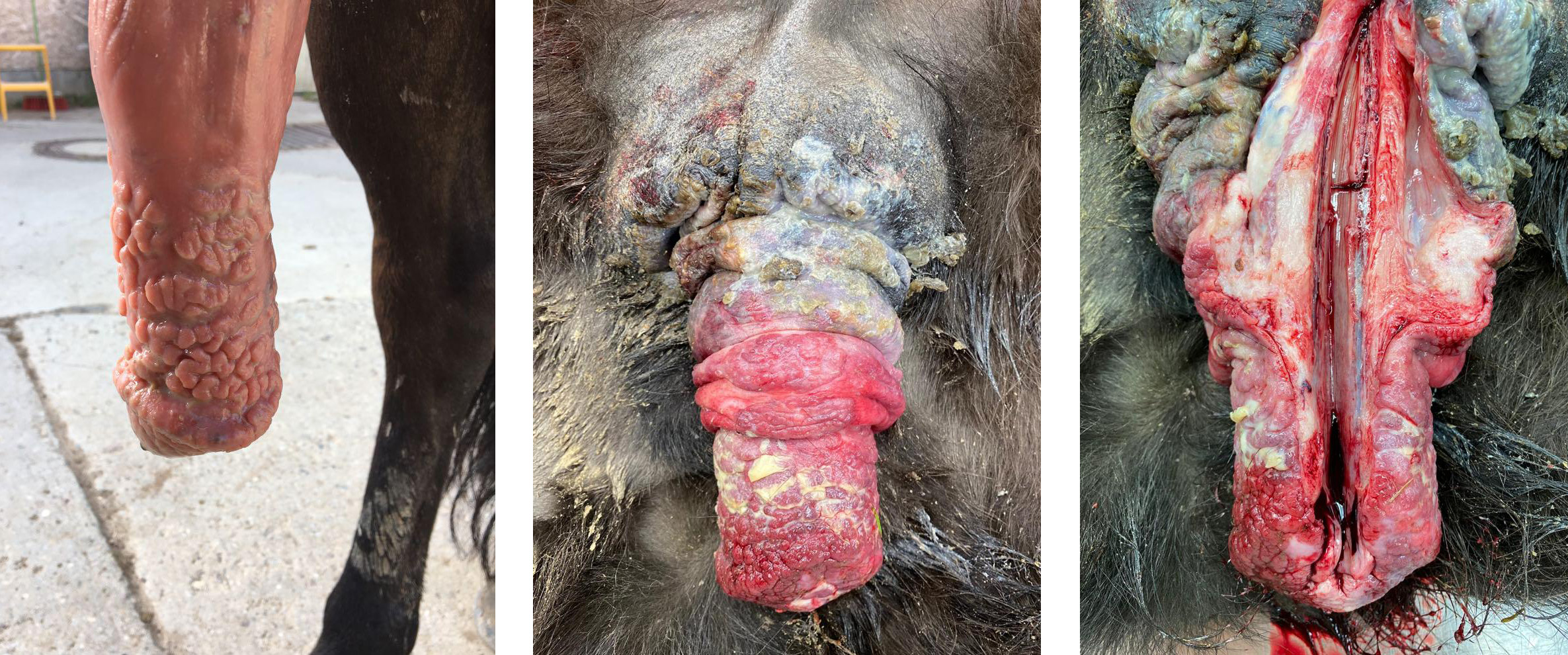
The horse has had penile lesions with multifocal mucosal thickenings and swelling for years.
Gross Pathology:
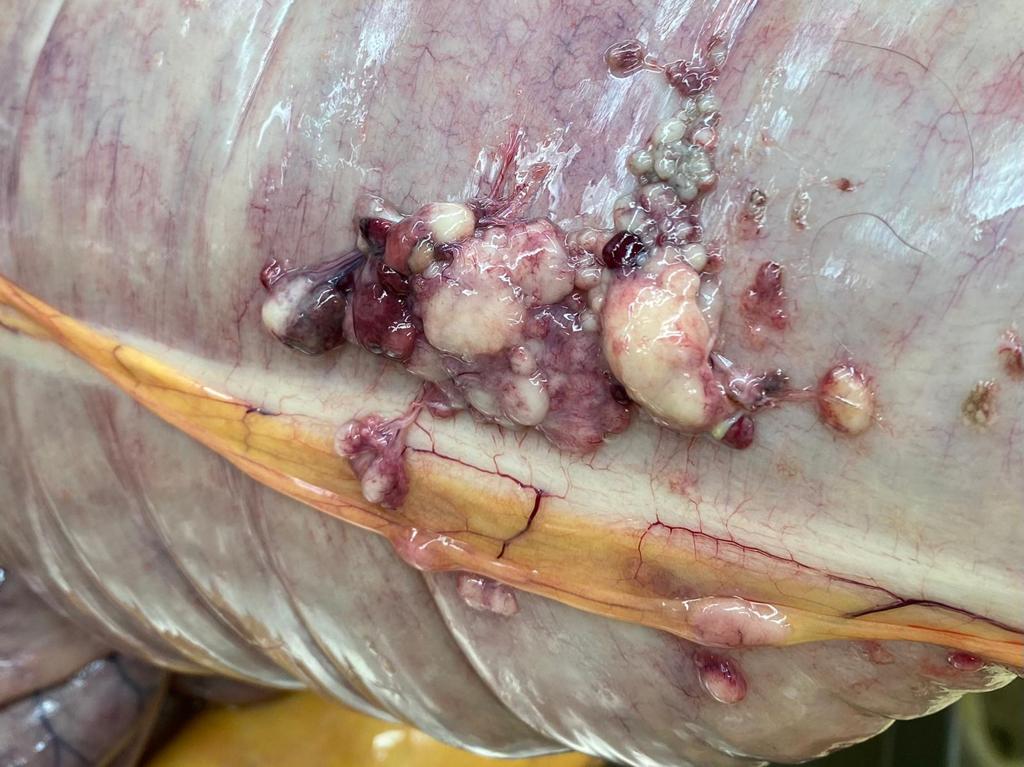
The penis was severely reddened and oedematous, the mucosa exhibited multifocal nodular thickenings and superficial yellow deposits. At the apex of the caecum, the mesentery of the large intestine and in the omentum there were multiple solid beige nodules about 1 to 3 cm in diameter.
Microscopic Description:
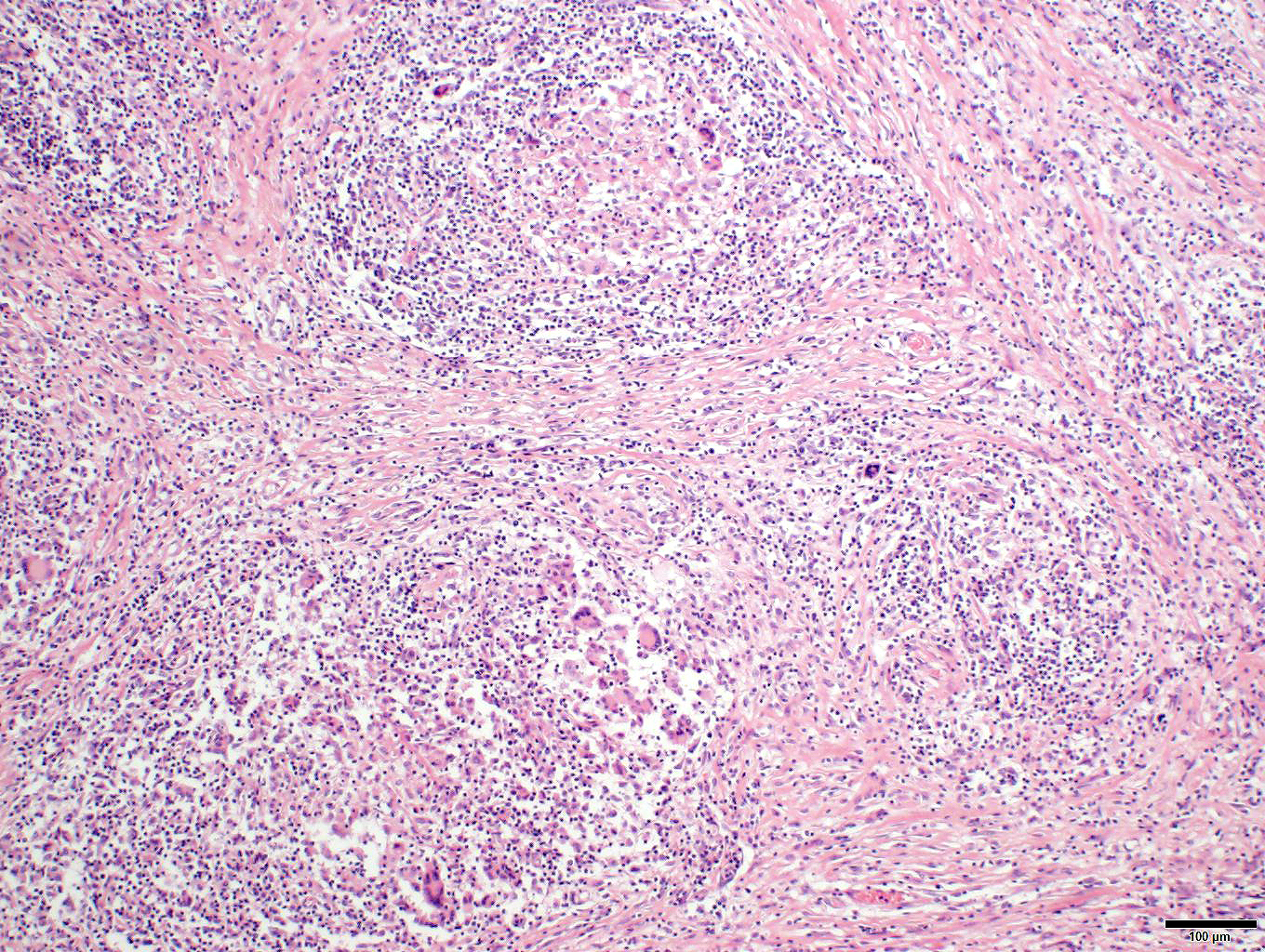
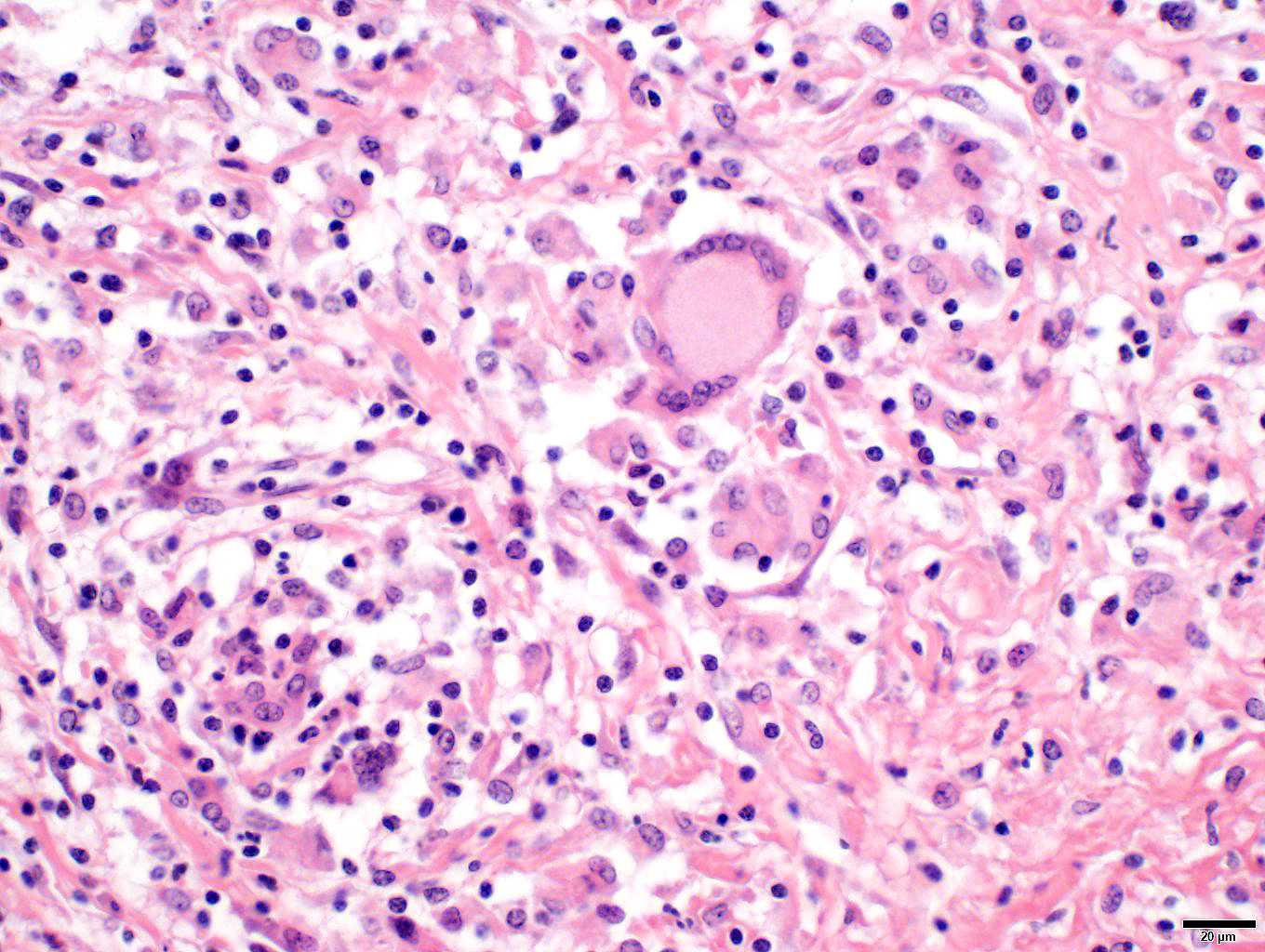
Penis/prepuce: The subepithelial connective tissue (submucosa) is severely expanded by a multifocal to coalescent, variable densely cellular inflammatory infiltrate consisting of high numbers of macrophages and lymphocytes, fewer numbers of plasma cells, and multinucleated giant cells (predominantly Langhans type). Lymphocytes often accompany the mucosa or surround deeper blood vessels (perivascular cuffing). Cells frequently form merging granulomas characterized by nodules with abundant central partially epithelioid macrophages and few degenerate neutrophils, surrounded by lymphocytes, plasma cells, and multiple multinucleated giant cells admixed with plump, reactive fibroblasts and small amounts of loose collagenous connective tissue (fibrosis). The overlying mucosa is thickened, forming irregular rete ridges (hyperplasia) and infiltrated by neutrophils, partially forming serocellular crusts. In some areas, the mucosa is lost (ulceration) and the submucosa expanded by increased numbers of small caliber vessels, accompanied by activated fibroblasts admixed with loose collagenous connective tissue (granulation tissue).
Additional special stains were used for histology:
Ziehl-Neelsen stain for acid fast bacteria: negative
PAS reaction: negative
Contributor’s Morphologic Diagnosis:
Penis/prepuce: Balanoposthitis, severe, chronic-active, multifocal to coalescing, granulomatous with epithelial hyperplasia, partial ulceration, granulation tissue formation, lymphocytic vasculitis and numerous multinucleated giant cells.
Lymph node: Lymphadenitis, mild, multifocal, chronic-active, granulomatous with numerous multinucleated giant cells.
Contributor’s Comment:
The pathological findings are characterized by a systemic granulomatous inflammation without indications of a specific cause as no particular pathogen related lesions were present. The picture is therefore consistent with Equine idiopathic systemic granulomatous disease (ISGD), also referred to as “Equine sarcoidosis”, “Equine generalized granulomatous disease”, “Equine systemic granulomatous disease”, “Equine histiocytic disease” or “Equine histiocytic dermatitis.”7
The etiology of ISGD is unknown, though it has similarities to human sarcoidosis which is presumed to be a multifactorial disease due to an abnormal host response to antigens.5 Etiological investigations failed to identify specific agents. Similar lesions were documented after ingestion of Vincia villosa ('hairy vetch') by horses and more likely cattle, but not all by ISGD affected horses had been exposed to it.9
Clinical onset is variable. Cutaneous lesions in ISGD may present as an exfoliative dermatitis or less commonly, as a nodular lesions.5,6 Additionally, internal organs are often involved in the course of a generalized disease with the lung, liver, gastrointestinal tract, spleen, kidney, bones, and central nervous system being affected in decreasing frequency. Although lymph nodes are often involved, a peripheral lymphadenopathy is mostly absent. In the case presented here, regional lymph nodes were enlarged and histopathology revealed multifocal sometimes nodular infiltrates by macrophages and giant cells were detectable. In generalized disease, most horses develop a wasting syndrome.6,7 Diagnosis is primarily done by exclusion of infectious diseases (like dermatophilosis, dermatophytosis), autoimmune diseases (like pemphigus foliaceus, systemic lupus erythematosus), allergic reactions (like cutaneous adverse drug reaction, contact dermatitis), miscellaneous conditions (like seborrhea, multisystemic eosinophilic epitheliotropic disease), neoplasia (like epitheliotropic lymphoma), and toxins (like hairy vetch).5 Characteristic pathohistological findings are so called sarcoidal granulomas affecting the skin or internal organs. Multinucleated histiocytic giant cells are typical and numerous.
Contributing Institution:
Department of Veterinary Pathology, Freie Universität Berlin
http://www.vetmed.fu-berlin.de/en/einrichtungen/institute/we12/index.html
JPC Diagnosis:
Mucous membrane, penis: Balanoposthitis, lymphohistiocytic, chronic, diffuse, moderate with marked epidermal hyperplasia and ulceration and lymphocytic perivasculitis.
Fibrovascular tissue (presumed peritoneum): Peritonitis, granulomatous, chronic, diffuse, severe.
JPC Comment:
Case 3 was challenging for participants in that the exact location sampled is not obvious from the slide alone. The presence of large, multinucleated giant cell macrophages was a helpful feature that led participants to consider equine sarcoidosis for this case. Because sarcoidosis is a diagnosis of exclusion, excluding other causes of granulomatous inflammation (e.g. fungal, mycobacterial, pythiosis, foreign body reaction, Actinobacillus) must be performed. In addition to the acid-fast stain performed by the contributor, we performed modified Gram stains (Brown-Brenn, Brown-Hopps), GMS, and PAS Light Green which were negative for organisms. IHC for IBA1, CD3, and CD20 were also helpful is establishing the distribution of these inflammatory cells and excluding T-cell rich B-cell lymphoma as a possibility as well. Previously, equid gammaherpesvirus 2 was implicated as a potential cause of this condition,3 which is a reasonable connection given that lymphohistiocytic inflammation is associated with gammaherpesviruses across species (e.g. malignant catarrhal fever). That stated, EHV-2 has not been a consistent finding in all cases of sarcoidosis and it is possible that the development of granulomatous inflammation may be the result of any number of antigens.8
Understanding of sarcoidosis is largely extended from human medicine, to include the entity name itself. Patients with sarcoidosis appear to have increased genetic susceptibility, to include variance in MHC class II genes1 such as HLA-DRB1 that is associated with acute sarcoidosis and Löfgren syndrome. M1 macrophages are activated by a highly polarized T helper 1 (Th1) cytokine milieu including IFN-γ and TNF-α which correlate with the Langhans multinucleated giant cells seen in our case. Interestingly, not all of these macrophages may be classically activated. Cytokine studies of sarcoidosis patients have identified macrophages with increased IL-13 expression (i.e. M2 macrophages) that may play a role in later stages of the disease.2 Additionally, M2 macrophages also form multinucleated giant cells (foreign body type), which were also observed in the present case.
As a final point, equine sarcoid should not be confused with sarcoidosis as they are entirely separate from one another. The term ‘sarcoid’ denotes a raised plaque or nodule in the skin (literally, sarcoma-like) which can be a feature of sarcoidosis, though other cutaneous presentations can include crusting and scaling.8 Equine sarcoids differ histologically from sarcoidosis in that they have increased density of dermal fibroblasts which form interlacing bundles and whorls within the dermis.4 Bovine papillomavirus type 1 and type 2 are associated with the development of equine sarcoids4 though the association of viruses in the development of sarcoidosis, if any, is uncertain.
References:
- Chen ES, Moller DR. Sarcoidosis--scientific progress and clinical challenges. Nat Rev Rheumatol. 2011 Jul 12;7(8):457-67.
- Locke LW, Crouser ED, White P, Julian MW, Caceres EG, Papp AC, Le VT, Sadee W, Schlesinger LS. IL-13-regulated Macrophage Polarization during Granuloma Formation in an In Vitro Human Sarcoidosis Model. Am J Respir Cell Mol Biol. 2019 Jan;60(1):84-95.
- Nolte LC, Rosiak M, Baechlein C, Baumgärtner W, Allnoch L. Equine Idiopathic Systemic Granulomatous Disease With Manifestation in the Cerebellum Associated With Equid Gammaherpesvirus 2. Journal of Equine Veterinary Science. 2020; (94):103225.
- Og?uszka M, Starzy?ski RR, Pierzcha?a M, Otrocka-Domaga?a I, Ra? A. Equine Sarcoids—Causes, Molecular Changes, and Clinicopathologic Features: A Review. Veterinary Pathology. 2021;58(3):472-482.
- Scott DW, Miller WH: Chapter 15 - Miscellaneous Skin Diseases. In: Equine Dermatology, eds. Scott DW, Miller WH, pp. 647-697. W.B. Saunders, Saint Louis, 2003
- Stannard AA. Immunologic diseases. Veterinary Dermatology 11: 163-178, 2000
- Van Oldruitenborgh-Oosterbaan MMS, Grinwis GCM: Equine sarcoidosis: clinical signs, diagnosis, treatment and outcome of 22 cases. Veterinary Dermatology 24: 218-e248, 2013
- Wimmer-Scherr CM & Schwarz, B.C. (2024) A narrative literature review of equine sarcoidosis. Equine Veterinary Education, 00, 1–8.
- Woods LW, Johnson B, Hietala SK, Galey FD, Gillen D: Systemic granulomatous-disease in a horse grazing pasture containing Vetch (Vicia sp). Journal of Veterinary Diagnostic Investigation 4: 356-360, 1992