Wednesday Slide Conference, Conference 9, Case 3
Signalment:
3.5 year-old, intact female, Drill, Mandrillus leucophaeus, non-human primate.
History:
The animal was kept in a zoo with access to indoor- and outdoor facilities. It was presented to the veterinarian due to a mass in the anterior chamber of the left eye. The left eye was removed and submitted for further investigation to the Institute of Veterinary Pathology at the Ludwig-Maximilians-Universität in Munich, Germany.
Gross Pathology:
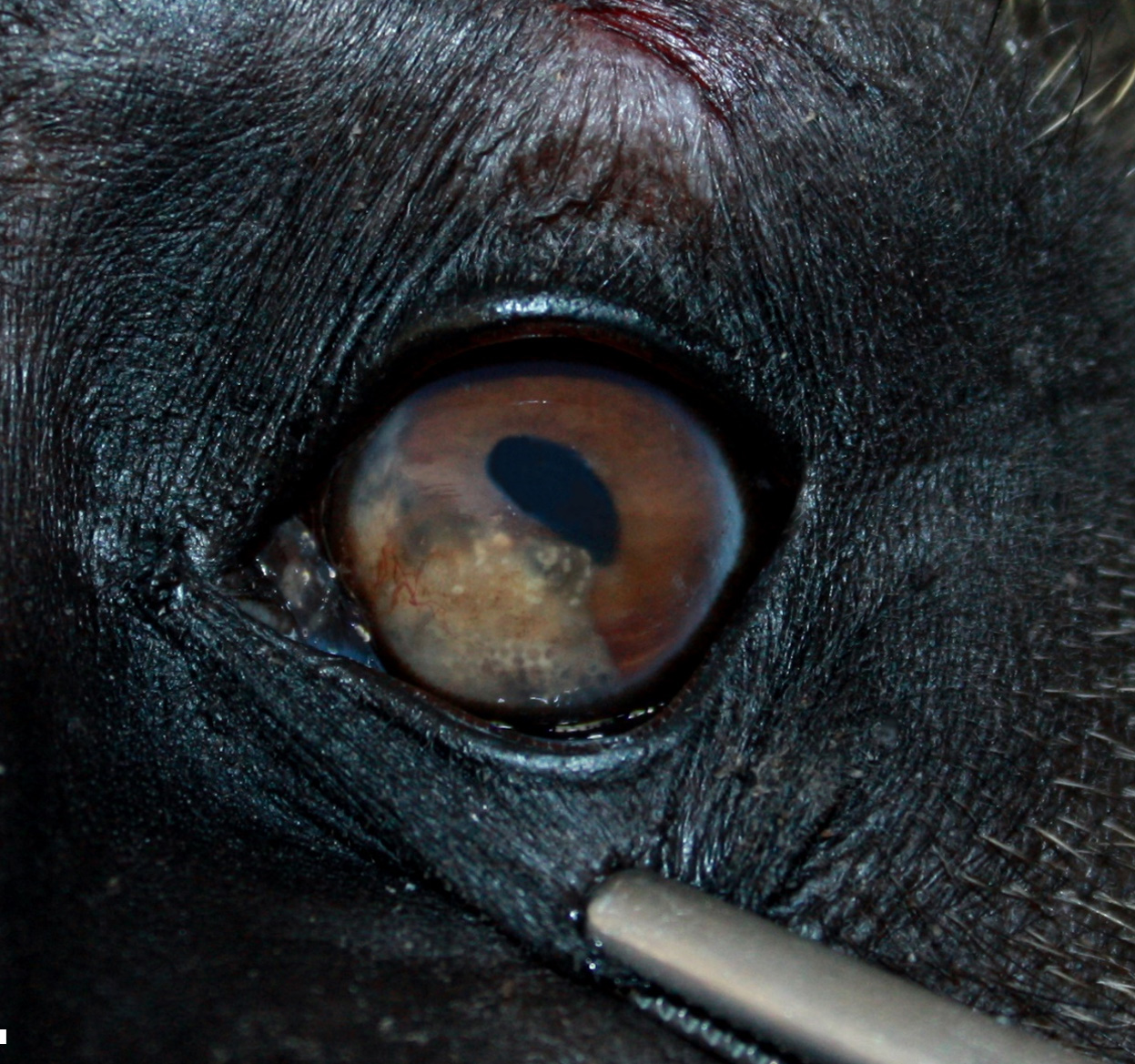
In situ-inspection of the left eye revealed a yellowish brown, and immovable mass filling the nasoventral part of the left anterior eye chamber. After removal of the eye and 24 hours of fixation with modified Davidson solution, the mass was further examined and trimmed. The mass measured ca. 1 cm x 0.8 cm x 0.3 cm and was confined to the iris and ciliary body. On cut section, the surface of the iris and ciliary body appeared solid to cystic. The lens was mildly displaced.
Laboratory Results:
Echinococcus multilocularis - PCR (lung, liver, eye): positive.
Echinococcus multilocularis ? Immunohistochemistry (eye): positive.
Microscopic Description:
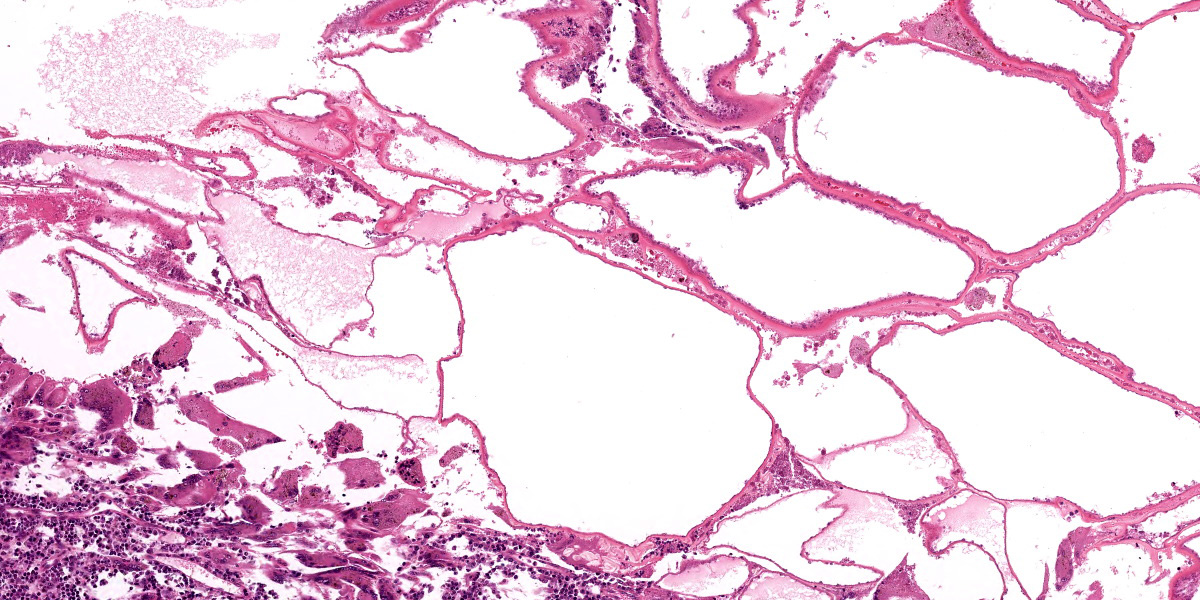
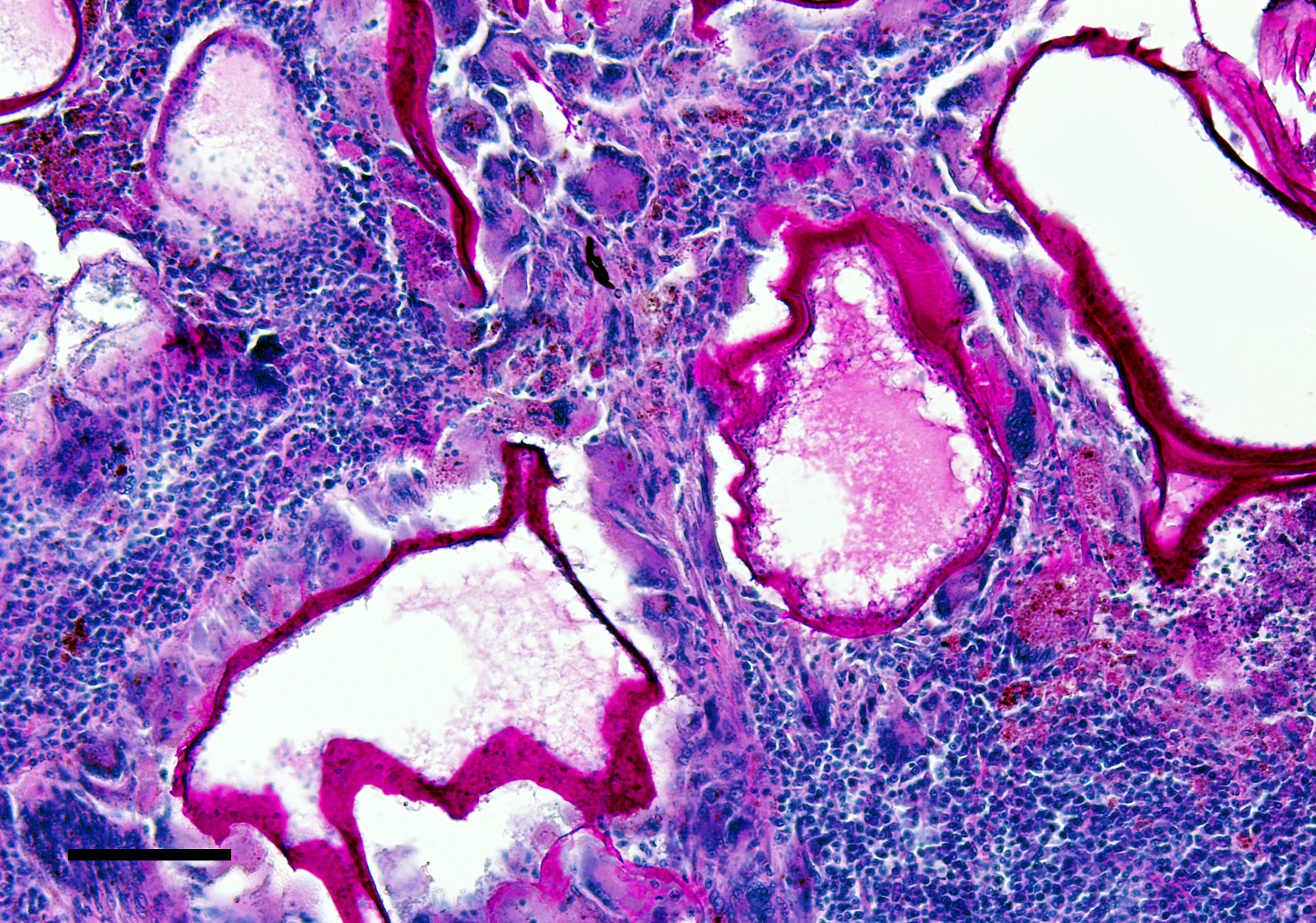
Left eye: The stroma of the iris and ciliary body is focally extensively expanded by a multiloculated hydatid cyst accompanied by marked inflammatory cell infiltration and necrosis. In particular, the iridociliary stroma is partly effaced by multiple intact or collapsed cystic spaces of different sizes (ranging from 15 to 1500 µm in diameter) surrounded by a 10-15 µm thin hyaline acellular laminated cyst wall (laminated layer). The inner part of the cyst wall is often lined by a monolayer of cuboidal cells (germinal layer). The cystic spaces are either optically empty or simply contain finely granular amphophilic material (non-fertile hydatid cyst). The cyst walls are surrounded by numerous multinucleated giant cells with up to 60 nuclei that are irregularly distributed within the cytoplasm (foreign-body giant cells), intermingled with large numbers of macrophages, lymphocytes, and plasma cells, and lower numbers of eosinophilic granulocytes. Intralesionally, there are multiple areas consisting of small cyst wall fragments and cellular debris characterized by loss of cellular detail, hypereosinophilia, and shrinked and fragmented nuclei (necrosis). The expanded iris adheres to the cornea (anterior synechia) and the associated part of the iridocorneal angle is closed.
Moderate to large numbers of plasma cells, lymphocytes, and macrophages are infiltrating the remaining portions of the iridociliary stroma, the trabecular meshwork, as well as the immediately surrounding portions of the choroidea, sclera, and cornea.
Additionally, ocular adnexa including the conjunctiva show multifocal infiltration of low to moderate numbers of lymphocytes and plasma cells.
Contributor?s Morphologic Diagnosis:
Left eye: Hydatid cyst, multiloculated, non-fertile, iridociliary, with marked chronic granulomatous and eosinophilic anterior uveitis with stromal necrosis, anterior synechia, and mild to moderate chronic lymphoplasmacytic scleritis and conjunctivitis.
Contributor?s Comment:
Echinococcus multilocularis (EM) belongs to the phylum Platyhelminthes, class Cestoda (tapeworms), and subclass Eucestoda. Within the order Cyclophyllidea it is further subordinated to the family Taeniidae. The larval stage of EM is the causative agent of alveolar echinococcosis (AE), a parasitic zoonotic disease of great medical and veterinary importance distributed in the northern hemisphere.2,8
The parasite?s life cycle usually involves various species of wild rodents, such as Arvicolinae and Cricetinae, and the lagomorph pika (Ochotona curzoniae) on the Tibetian plateau of China as intermediate hosts. Depending on the epidemiological setting, wild or domestic canid and felid species such as red or arctic foxes, jackals, wolves, dogs, and cats serve as the parasite's definitive hosts.2,3,7,8 Moreover, dogs can simultaneously act as definitive and intermediate hosts.2
The definitive host harbors the egg-producing adult form of EM (tapeworm) within the intestine and eggs or the egg containing last segment of the worm (gravid proglottid) are shed via feces. The infective eggs are dispersed in the environment, where they can survive approximately 1 year in a suitable, moist environment at lower temperatures, but they are sensitive to desiccation and high temperatures.3 Once the eggs are ingested by the intermediate host, they hatch in the small intestine. The hexacanth larva (oncosphere) is released and penetrates the gut wall. It then passes through the portal and lymphatic vessels, eventually reaching various organs in the intermediate host's body.8,11 Within the affected organs of the intermediate host, the oncosphere develops into a metacestode, which is a larval stage characterized by multilocular vesiculated cyst formation. This disease is called ?alveolar echinococcosis? due to the appearance of these cysts.1
These cysts have an outer laminated layer and an inner germinal layer, and in case of fertile cysts, the germinal layer produces brood capsules containing protoscoleces.4 The life cycle is completed when the definitive host consumes the organs of the intermediate host that contain fertile metacestodes. Within the intestine, the protoscoleces develop into adults. The prepatent period between ingestion of protoscoleces and the production of eggs by the matured tapeworm typically ranges from 4 to 7 weeks.8
Aberrant intermediate host animals and humans can also become infected with the metacestode stage by accidental ingestion of oncosphere-containing eggs.3 However, aberrant intermediate host animals do not play a role in the transmission cycle and include horses, cattle, domestic and wild pigs, nutria (Myocastor coypus), and several species of nonhuman primates (NHP).3,11 Infection with EM metacestodes is reported from a variety of NHP, including gorilla (Gorilla gorilla), orangutan (Pongo pygmaeus), rhesus monkey (Macaca mulatta), cynomolgus monkey (Macaca fascicularis), lion-tailed macaque (Macaca silenus),1,9 Japanese monkeys (Macaca fuscata),5 and ring-tailed lemur (Lemur catta).6 After a large outbreak in a German breeding enclosure with three different affected NHP species, cynomolgus monkeys were considered to be at higher risk, and therefore, presumably are more susceptible to infection than other NHP species. However, it is important to note that NHP in zoological gardens and institutional colonies, particularly in endemic regions, are at risk of becoming infected with EM.9
AE is of increasing concern globally due to the geographical spread of EM, its increasing prevalence in animals from endemic areas, the absence of a vaccine, and the lack of active control measures to prevent the infection.2
Once the aberrant intermediate host is infected, the metacestode infiltration and proliferation progress silently (in humans, over the course of 5 to 15 years), causing AE, a chronic and complex disease with a devasting clinical condition and high fatality rate if left untreated.2,8
AE primarily affects the liver in intermediate hosts as well as aberrant intermediate hosts and behaves like an infiltrative and eventually metastasizing tumor with further infiltration of tissues close to the liver or dissemination via blood and lymphatic vessels.2,8,11 Less frequently than the liver are lungs, brain, bones, or any other organ of the (aberrant) intermediate host affected.9,11 Immune suppression exacerbates disease progression.2,8
In the present case, a full body necropsy was carried out after the evaluation of the eye, and a subsequent histological examination of further organs failed to identify any additional diseases or evidence of immunosuppression. However, besides the earlier diagnosed intraocular invasion, multiple organs were affected by larval infection, including both common and less common tissues including liver, kidneys, lungs, heart, lymph nodes, skeletal muscles, and brain. The laminated layer of the cysts displayed consistently positive PAS-reactivity. In addition, none of the cysts in this animal contained brood capsules with protoscoleces (non-fertile cysts). In general, fertility of EM cysts is common in susceptible hosts, where it is reached within 2?4 months. But it is far rarer (<20%) in resistant hosts, such as humans, or most domestic animals8. Aside from the absent brood capsules and protoscoleces, typical calcareous corpuscles were also not seen in the present case. These corpuscles can be very helpful for identification purposes in histological sections and appear as basophilic to clear ovals with a concentric ringed appearance, but they may ?dissolve? in fixation or histological processing.4
In human as well as veterinary medicine, the gold standard for AE diagnosis is the identification of parasite structures/genome in samples obtained invasively. Imaging techniques demonstrate characteristic features of the lesions, while serology is complementary.2,3
In 2020, an international consensus on terminology to be used in the field of echinococcosis was published. To harmonize echinococcosis terminology on sound scientific and linguistic grounds, the World Association of Echinococcosis launched a Formal Consensus process. The main achievements of this process were: (1) an update of the current nomenclature of Echinococcus spp.; (2) an agreement on three names of diseases due to Echinococcus spp.: Cystic Echinococcosis, Alveolar Echinococcosis, and Neotropical Echinococcosis, and the exclusion of all other names; (3) an agreement on the restricted use of the adjective ?hydatid? to refer to the cyst and fluid due to E. granulosus sensu lato; and (4) an agreement on a standardized description of the surgical operations for CE, according to the ?Approach, cyst Opening, Resection, and Completeness? (AORC) framework. In addition, 95 ?approved? and 60 ?rejected? terms were listed.10 In detail, the term ?hydatid? (as noun and as adjective) as well as the term ?cyst? should be restricted (by usage, not strictly by definition) to the metacestode of E. granulosus sensu lato causing cystic echinococcosis. It should not be used for the metacestode of E. multilocularis. This consensus was processed and published in order to avoid confusion between human diseases caused by the various species of Echinococcus. ?Hydatid? should further not be used to designate anything relating to AE or neotropical echinococcoses caused by E. vogeli or E. oligarthra. Among all the specialists working in the field of echinococcosis, such as in experimental models, veterinary pathologists are also asked to use the term (echinococcal) ?microcyst? in AE lesion instead of the term ?hydatid cyst.?10
Further parasites that can be found incidentally in the animal eye:12
- Echinococcus in nonhuman primates
- Cysticercus in swines
- Microfilariae of Elaeophora schneideri in elks causing occlusive vasculitis with multifocal ischemic chorioretinitis and optic neuritis in elks
- fortuitous localization of larvae of Toxocara canis or other ascarids, Angiostrongylus vasorum and Dirofilaria immitis in canine species, associated with uveitis
- Larvae of Onchocerca cervicalis and adults of Setaria spp. in horses
- Fly larvae in various species causing ophthalmomyasis due to intraocular migration
- Larvae of Diplostomum spathaceum in fishes
The lens fluke (Diplostomum spathaceum) is the only known specific intraocular parasite. This parasite is primarily found in the eye lenses of fishes. After penetrating the fish's skin, the Diplostomum larvae migrate to the lens of the eye with remarkable speed and specificity. The presence of numerous larvae within the lens can lead to the development of cataracts. These larvae are ingested by fish-eating birds, which completes the life cycle of the parasite.12
Contributing Institution:
Institute of Veterinary Pathology at the Centre for Clinical Veterinary Medicine
Ludwig-Maximilians-Universität München Veterinärstrasse 13
D-80539 Munich, Germany
https://www.patho.vetmed.uni-muenchen.de
JPC Diagnosis:
Eye: Panuveitis, granulomatous, focally extensive, severe, with anterior synechiae, lymphohistiocytic conjunctivitis, and hydatid cyst.
JPC Comment:
The contributor provides a detailed writeup on Echinococcus that accompanies great gross photos and an interesting histologic presentation.
Conference participants discussed ancillary changes within the eye which included closure of the iridocorneal angle and mild corneal edema beyond the other changes the contributor notes. Though there was a mild amount of fibrin lining the contralateral iridocorneal angle, this animal likely did not have sufficient deficit in aqueous humor drainage to cause glaucoma ? comparison to the optic nerve, retina, and Descemet?s membrane were helpful checks in this case to confirm that histologically. The lack of calcareous corpuscles or protoscoleces in histologic section of this infertile microcyst frustrated some conference participants, though recognition of the thick cyst wall (largely host derived) and germinal lining epithelium were key features. As the contributor hints at, the long duration of this infection in a resistant host makes identifying these features important as they may be the only specific ones that point to Echinococcus in these diagnostic cases. For comparison to a more acute infection in a Barbary ape, see VSPO D-P28A for examples of all representative organism features.
This case highlights the potential confusing nomenclature that arises with the shift (and adoption) of new terms to describe entities. Adoption of ?microcyst? and alveolar echinococcosis stymied some of the participants who felt that the morphologic diagnosis should retain the historical term as this change enters veterinary literature and awareness. In solidarity with the contributor, we kept the term hydatid cyst for this conference, but invite readers to explore the reference by Vuitton et al10 that the contributor helpfully provides.
References:
- Bacciarini L, Gottstein B, Pagan O, Rehmann P, Gröne A. Hepatic alveolar echinococcosis in cynomolgus monkeys (Macaca fascicularis). Vet Pathol. 2004;41:229-234.
- Casulli A, Barth TFE, Tamarozzi F. Echinococcus multilocularis. Trends Parasitol. 2019;35:738-739.
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107-135.
- Gardiner CH, Poynton SL. Morphological characteristics of cestodes in tissue section. In: An Atlas of Metazoan Parasites in Animal Tissues. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology; 2006:50-55.
- Kishimoto M, Yamada K, Yamano K, et al. Significance of imaging features of alveolar echinococcosis in studies on nonhuman primates. Am J Trop Med Hyg. 2009;81:540-544.
- Kondo H, Wada Y, Bando G, Kosuge M, Yagi K, Oku Y. Alveolar hydatidosis in a gorilla and a ring-tailed lemur in Japan. J Vet Med Sci. 1996;58:447-449.
- Oksanen A, Siles-Lucas M, Karamon J, et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta-analysis. Parasit Vectors. 2016;9: 519.
- Tamarozzi F, Brunetti E, Vuitton D. Echinococcosis. In: Fabrizio B, ed. Helminth Infections and their Impact on Global Public Health. 2nd ed. Cham: Springer; 2022:257-312.
- Tappe D, Brehm K, Frosch M, et al. Echinococcus multilocularis infection of several Old World monkey species in a breeding enclosure. Am J Trop Med Hyg. 2007;77:504-506.
- Vuitton DA, McManus DP, Rogan MT, et al. International consensus on terminology to be used in the field of echinococcoses. Parasite. 2020;27:41.
- Wen H, Vuitton L, Tuxun T, et al. Echinococcosis: Advances in the 21st Century. Clin Microbiol Rev. 2019;32.
- Wilcock BP, Njaa BL. Special Senses. In: Maxie MG, ed. Jubb, Kennedy & Palmer's Pathology of Domestic Animals.: Volume 1. 6th ed. St. Louis, Missouri: Elsevier; 2016:407-508.e402.