Wednesday Slide Conference, Conference 20, Case 1
Signalment:
Approximately 5-month-old, female, cherry shrimp (Neocaridina davidi)
History:
This shrimp was one of 10 ornamental dwarf shrimps housed in a 28-gallon freshwater aquarium and was found dead with no noted premonitory signs. The death of this shrimp occurred one month after a complete water change from tap water to distilled and re-mineralized water although standard water acclimation procedures were followed.
Gross Pathology:
External examination revealed numerous fine, light green to yellow, elongate to club-shaped, up to 1 mm long structures projecting outward along the interpleopodal spaces.
Microscopic Description:
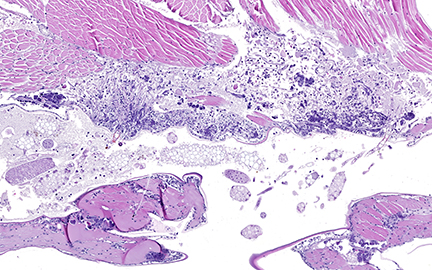
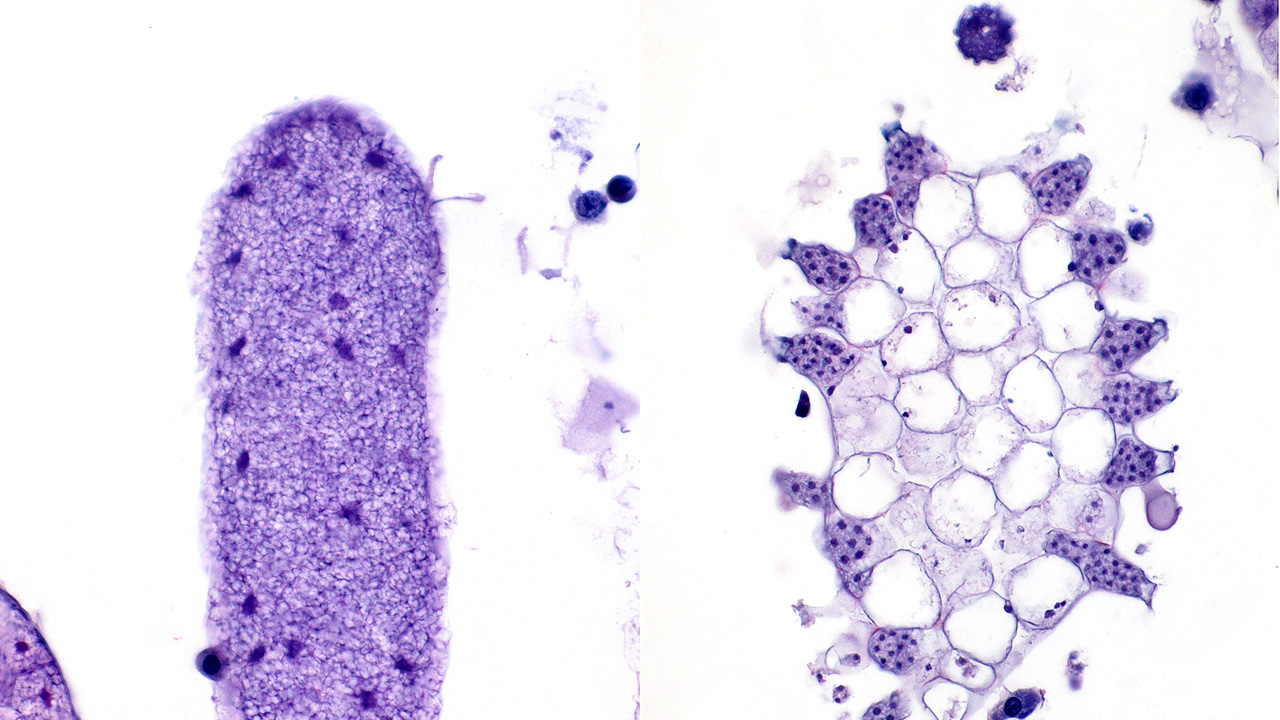
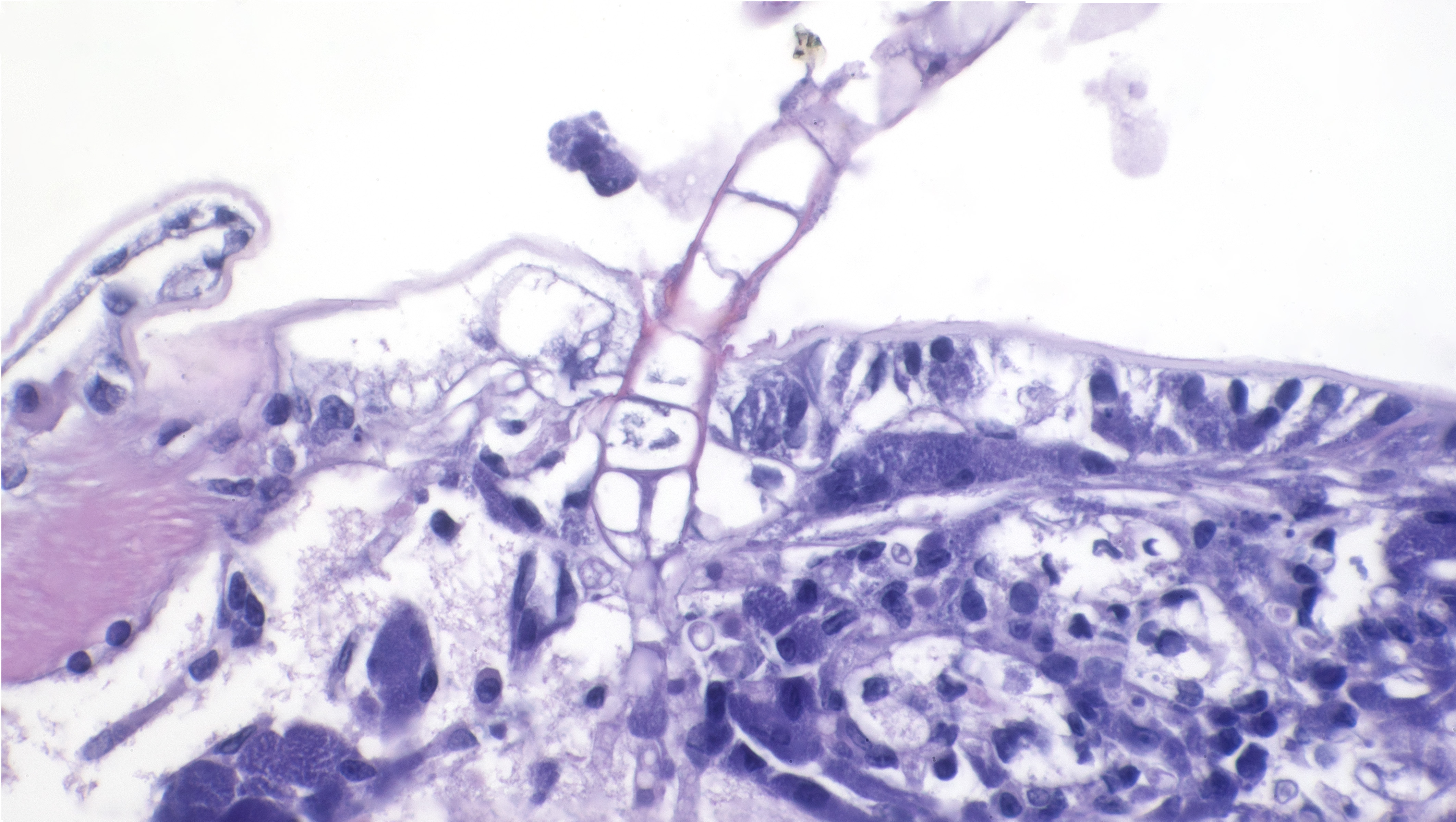
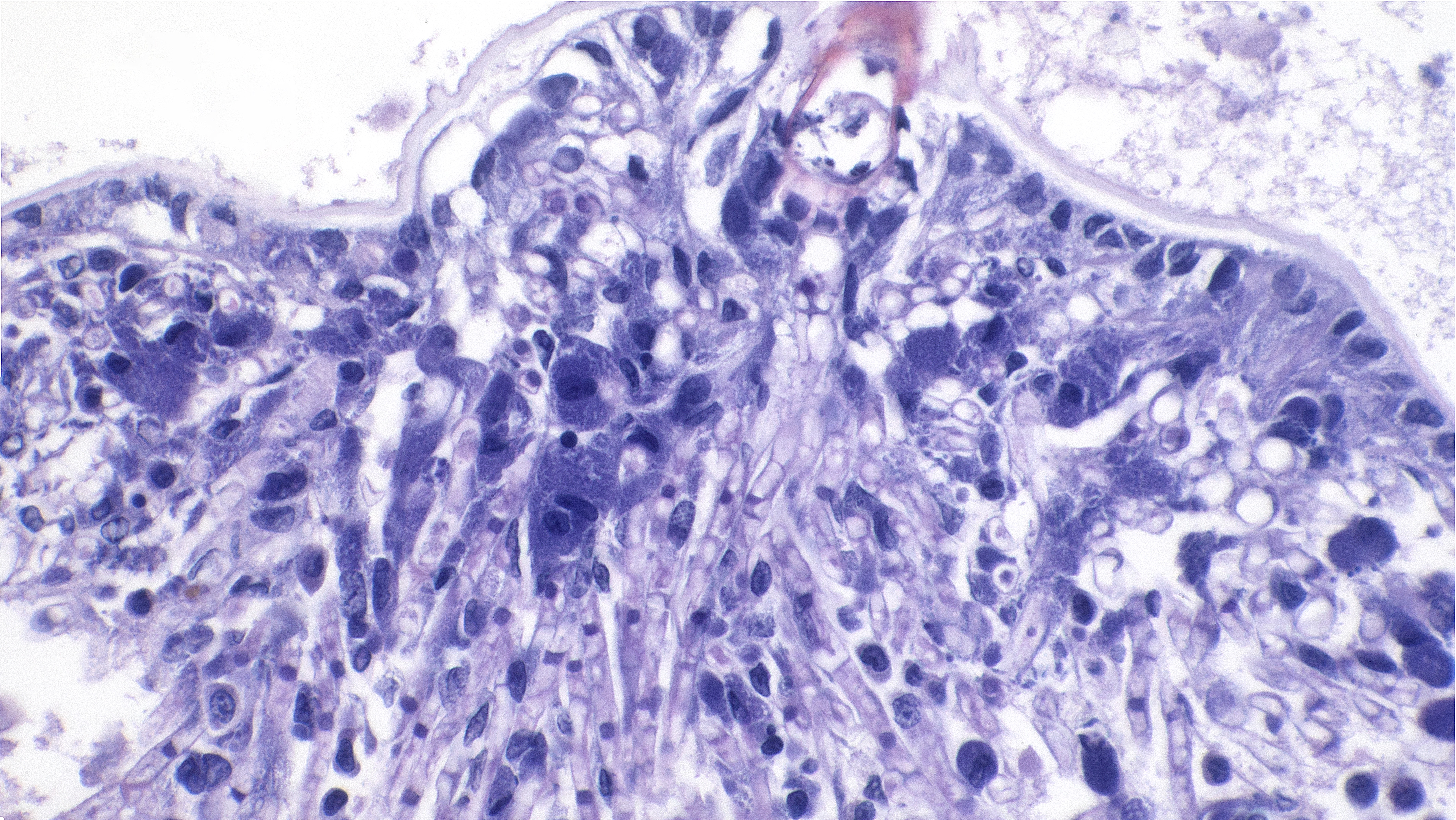
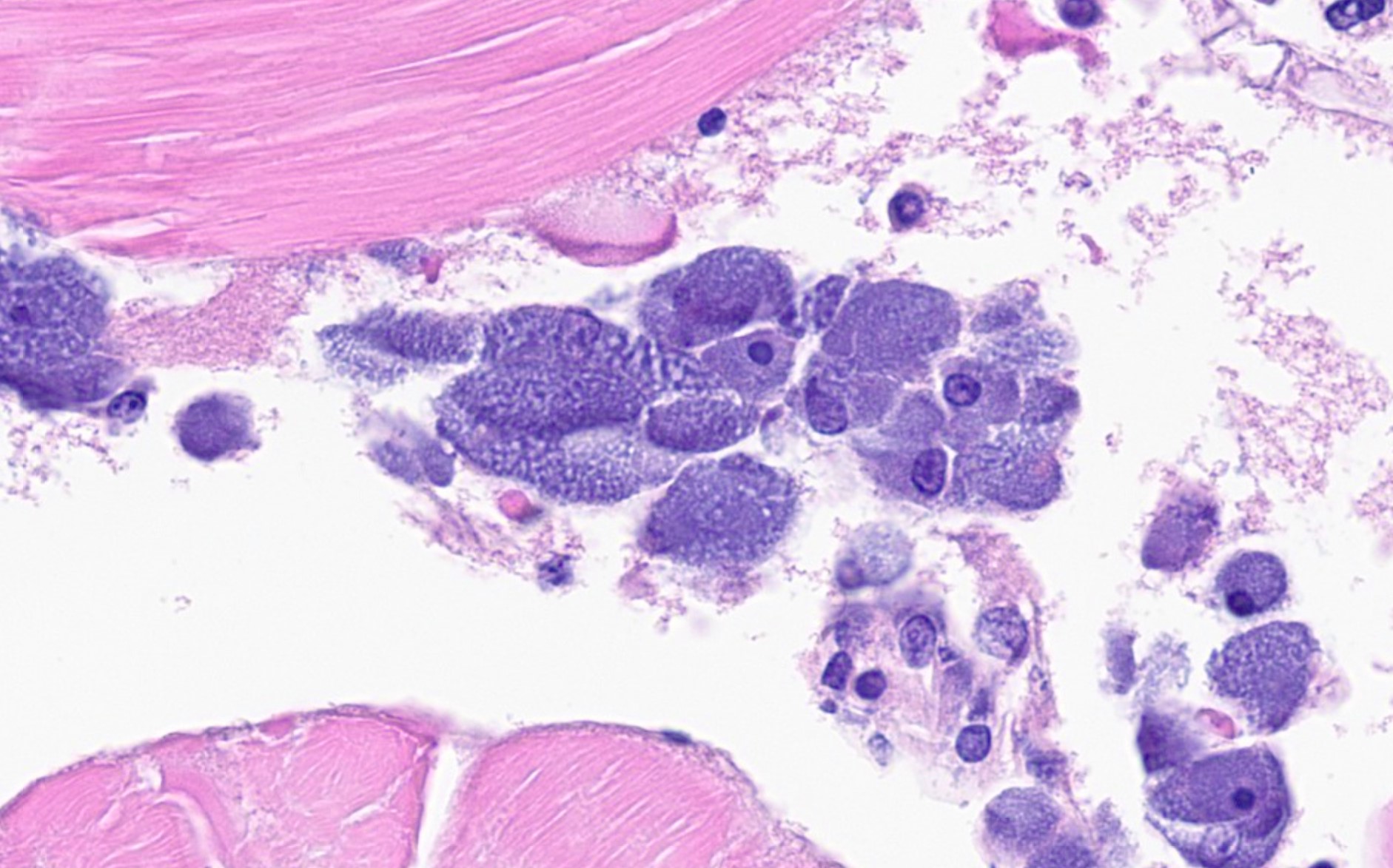
Sagittal sections of the entire shrimp were available for histologic review. Many thalli were protruding externally within the pleopodal regions and consisted of cuticular perforating multinucleated basal cells that measured 20 µm in diameter and externally branching erect filaments that ranged from 8 to 15 µm in diameter with numerous terminal zoosporangia of varying maturational stages. Fully sporulated zoosporangia measured up to 800 µm in length and 100 µm in diameter and consisted of numerous peripherally located zoospores. The perforating basal cells were associated with a branching network of hyphal-like rhizoids that infiltrated the epidermis and subcutis, ranged from 4 to 8 µm in diameter, were uninucleated and partitioned, and occasionally contained granular material highlighted by a Grocott methenamine silver stain (GMS). The subcutaneous tissue was markedly expanded by hemolymph and heavily infiltrated by predominately granulated hemocytes. Rhizoids and associated inflammatory infiltrates extended to and surrounded the ventral nerve cord and multifocally (but minimally) invaded the adjacent abdominal flexor musculature. All observed algae forms were strongly highlighted by a GMS stain. Similar uninucleated and partitioned, short rhizoids were also circulating within the hemolymph of the ventral cephalothorax, hepatopancreas, and around the midgut. Within these areas, pooled hemolymph contained numerous circulating hemocytes.
Contributor’s Morphologic Diagnosis:
Interpleopodal subcuticular invasive and circulating rhizoids, numerous, with external zoosporangia and infiltrating granulated hemocytes
Contributor’s Comment:
The external thallus portion consisting of cuticular perforating basal cells, branching erect filaments, and terminal zoospores as well as subcutaneous rhizoids within the pleopod area are morphologically consistent with Cladogonium sp. Cladogonium sp. is a parasitic epibiotic green algae of East Asian freshwater shrimp. It was first described in Paratya improvisa in 19501, then described in Macrobranchium longipes in 19712, and since then has been well characterized in Neocaridina davidi.4,5 Cladogonium sp. is an important cause of epibiont-related morbidity and mortality in aquacultured and captive Neocaridina davidi. Classification of C. ogishimae and C. kumaki sp. nov. has primarily been based on morphology with limited molecular characterization grouping Cladogonium sp. within the order Trentepohliales although still widely accepted as belonging to the order Cladophorales and family Pithophoraceae.1,5
Neocaridina davidi are freshwater dwarf shrimp that are native to Taiwan and are heavily bred for the aquatic pet trade industry due to their popular small size, intense coloration, and large diversity. The lack of readily available veterinary care and scientific literature on this agent has resulted in widespread misinformation amongst shrimp hobbyists.
The common misnomer for Cladogonium sp. is “green shrimp fungus” and has widely been confused with Ellobiopsidae. Ellobiopsidae is a small diverse group of protists that are closely related to dinoflagellates and are primarily ectoparasites of marine pelagic crustaceans (krill) and freshwater copepods and cladocerans (water fleas).3 Other well recognized epibionts of Neocaridina davidi and their preferred anatomical location include 1) Rotifera found along the rostrum and antennas; 2) Stentor sp. along the rostrum and antennas; 3) Scutariella japonica along the rostrum, pleopods, and pereiopods; 4) Monodiscus kumaki sp. nov. along the rostrum, pleopods, and pereiopods; 5) Saprolegnia sp. along the pleopods and uropods; 6) Holtodrilus truncates along the rostrum, branchial chambers, pleopods, and pereiopods; and 7) Vorticella sp. along the chelipeds, pereiopods, pleopods, and uropods. 4,5 Of these, Cladogonium ogishimae, Saprolegnia sp., and Scutariella japonica are known to exhibit parasitic lifestyles. 4
Importation of Neocaridina davidi and their epibionts is considered the main route of spread.4,5 It is suspected that following rhizoid colonization of the cuticular epidermis and subcutis along the interpleopodal region, basal cells perforate the cuticle and branch externally as erect filaments with terminal zoosporangia.1 Ciliated zoospores are then released into the environment by the mature zoosporangia. These contain chloroplasts, which impart the grossly observed green coloration and is evident in the advanced stage of infection. 6 All algal forms stain strongly with Grocott methenamine silver stain. Death is thought to be due to debilitation, potentially related to involvement of the ventral nerve cord, molting complications, and/or secondary infections.1 Systemic infections, as was seen in this case, have not been previously described. The possibility of a systemic coinfection with Saprolegnia parasitica was considered; however, the circulating short rhizoid segments are morphologically identical to those within the pleopod area with branching, partitioning, and uninucleation and are morphologically distinct from Saprolegnia sp. filaments.
Diagnosis of Cladogonium sp. infections is largely based on observation of the characteristic light green elongate club-shaped structures projecting outward in the pleopodal area, which are, again, evident in advanced infections as the erect filaments and immature zoosporangia largely lack chloroplasts. The prognosis is poor for heavily parasitized shrimp, which constitute the source of infection for other shrimp in the environment. While algaecides are effective at eliminating this agent by substituting copper for magnesium within chlorophyll, shrimp are exquisitely sensitive to copper toxicity and would be unlikely to survive treatment doses required to kill algae.1 Water quality management, quarantining, and reduction of stressors such as temperature fluctuations are all important in preventing and managing outbreaks of this disease.1 In our case, this disease manifested approximately one month after a 100% water change from tap water to distilled and re-mineralized water. During the water acclimation period, there was an ammonia spike in the temporary holding tank, which may have predisposed this shrimp to succumb to a preexisting parasitic infection. Considering that there were no additions or other changes to the tank since acquisition of the shrimp, an asymptomatic carrier state was suspected although could not be proved. No additional deaths were observed following prompt removal of the dead shrimp, and the remaining shrimps have prolifically bred under stable water and temperature parameters.
Contributing Institution:
Department of Pathobiological Sciences and Louisiana Animal Disease Diagnostic Laboratory, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
https://www.lsu.edu/vetmed/laddl/
https://www.lsu.edu/vetmed/pbs/index.php
JPC Diagnosis:
- Body as a whole: Hemocytosis, chronic, diffuse, severe with hemocytic and multisystemic cytoplasmic bacterial inclusions (rickettsia-like organisms)
- Ventral carapace: dermatitis, necrotizing, acute, regionally extensive, mild with invasive algae
JPC Comment:
This week’s moderator was Prof. Karen Terio from the University of Illinois who selected four exotics/wildlife cases to test conference participants on classic entities as well as several new ones. This first case features an ouotstanding, meticulously prepared sagittal section of a cherry shrimp. Preservation of major anatomic features7 such as the eye, anterior ganglion, antenna, gills, tail fin, and pleopods facilitates slide orientation and interpretation of lesions.
We enjoyed discussing the pathogenesis of this case. The most substantial finding for mortality was likely the rickettsia-like organism (RLO) infection, which resulted in numerous bacterial inclusions within the cytoplasm of hemocytes throughout the hemolymph. Other cells (particularly the epidermis) also had inclusions, though less frequently. Rickettsia-like organisms are primary pathogens of invertebrates and given the severity of inflammation, RLOs were likely the main cause of the decline seen in this case. Rickettsia are obligate intracellular bacteria. Infections with RLOs are widely reported in crustaceans. Regardless of the crustacean host, disease caused by RLOs has typical histologic findings, namely bacterial inclusions in hemocytes throughout the body and less commonly in other tissues, as seen in this case.
Given the limited distribution of the algal infection, participants suspected that the algal lesion was likely secondary to generalized debilitation from RLO infection, though two separate primary pathogenic processes are possible. We did not speculate on the exact algae involved (lacking ancillary confirmation) as microhabitat and epibiont-host relationships are not entirely exact,5 though the shape and distribution is appropriate for Cladogonium.
Finally, participants also discussed the nature of epibiosis. Epibiotic organisms live on the surface of another organism and this relationship can be harmless (neutral), commensalistic (both benefit), or parasitic (one benefits at the others expense). Such relationships have the potential to directly impact organismal coloration, health, and reproduction which all have significant economic impacts.5 In the present case, this relationship bests fits with a parasitic one. It is possible that entry of thalli (direct penetration) and previous areas of carapace damage both facilitated Cladosporium development. That the nine other conspecifics of this shrimp were not affected points to this host-epibiont relationship being fairly muted (or at least tolerable), though Cladogonium should still be viewed as a pathogen nonetheless.
References:
- Bauer J, Jung-Schroers V, Teitge F, Adamek M, Steinhagen D. Association of the alga Cladogonium sp. with a multifactorial disease outbreak in dwarf shrimp (Neocaridina Davidi). Diseases of Aquatic Organisms. 2021; 146:107-115.
- Hirose H, Akiyama M. A Colorless, Filamentous Chlorophyceous Alga, Cladogonium ogishimae Gen. et Sp. Nov., Parasitic on Fresh-water Shrimps. The Botanical Magazine Tokyo. 1971; 84(993):137-140.
- Konovalova GV. Parasitic Dinoflagellates and Ellobiopsids (Ellobiopsidae) of the Coastal Waters of the Sea of Japan. Russian Journal of Marine Biology. 2008;34(1):28-37.
- Maciaszek R, Kamaszewski M, Stru?y?ski W, ?apa P. Epibionts of ornamental freshwater shrimps bred in Taiwan. Annals of Warsaw University of Life Sciences - SGGW - Animal Science. 2018;57(2):133-142.
- Maciaszek R, Swiderek W, Prati S, Huang CY, et al. Epibiont Cohabitation in Freshwater Shrimp Neocaridina davidi with the Description of Two Species New to Science, Cladogonium kumaki sp. nov. and Monodiscus kumaki sp. nov., and Redescription of Scutariella japonica and Holtodrilus truncates. Animals. 2023;13(1616):1-22.
- Matsuyama-Serisawa K, Imai T, Nakaso M, Serisawa Y. Reconfirmation of Cladogonium (Chlorophyta, Cladophoraceae) being Ectoparasitic on Freshwater Shrimp. Japanese Journal of Phycology (Sôrui). 2014; 62:1−6.
- Smolowitz R. Arthropoda: Decapoda. In: LaDouceur EEB, ed. Invertebrate histology. Wiley-Blackwell; 2021: 277-299.