Wednesday Slide Conference, Conference 20, Case 2
Signalment:
Adult female betta fish (Betta splendens)
History:
This animal was wild-caught in Thailand and singly housed at the Wake Forest University Reynolda Campus for use in an experimental study evaluating differences in personality, behavior, and locomotion between wild and domestic betta fish. About 5 weeks after arrival from Thailand it appeared thin, but was observed consuming normal amounts of food. The animal hid in the tank and was found dead approximately 8 hours later. Three other female wild-caught betta fish in this cohort were also found dead. All domestically purchased male and female betta fish and male wild-caught betta fish in this cohort had no clinical signs.
Gross Pathology:
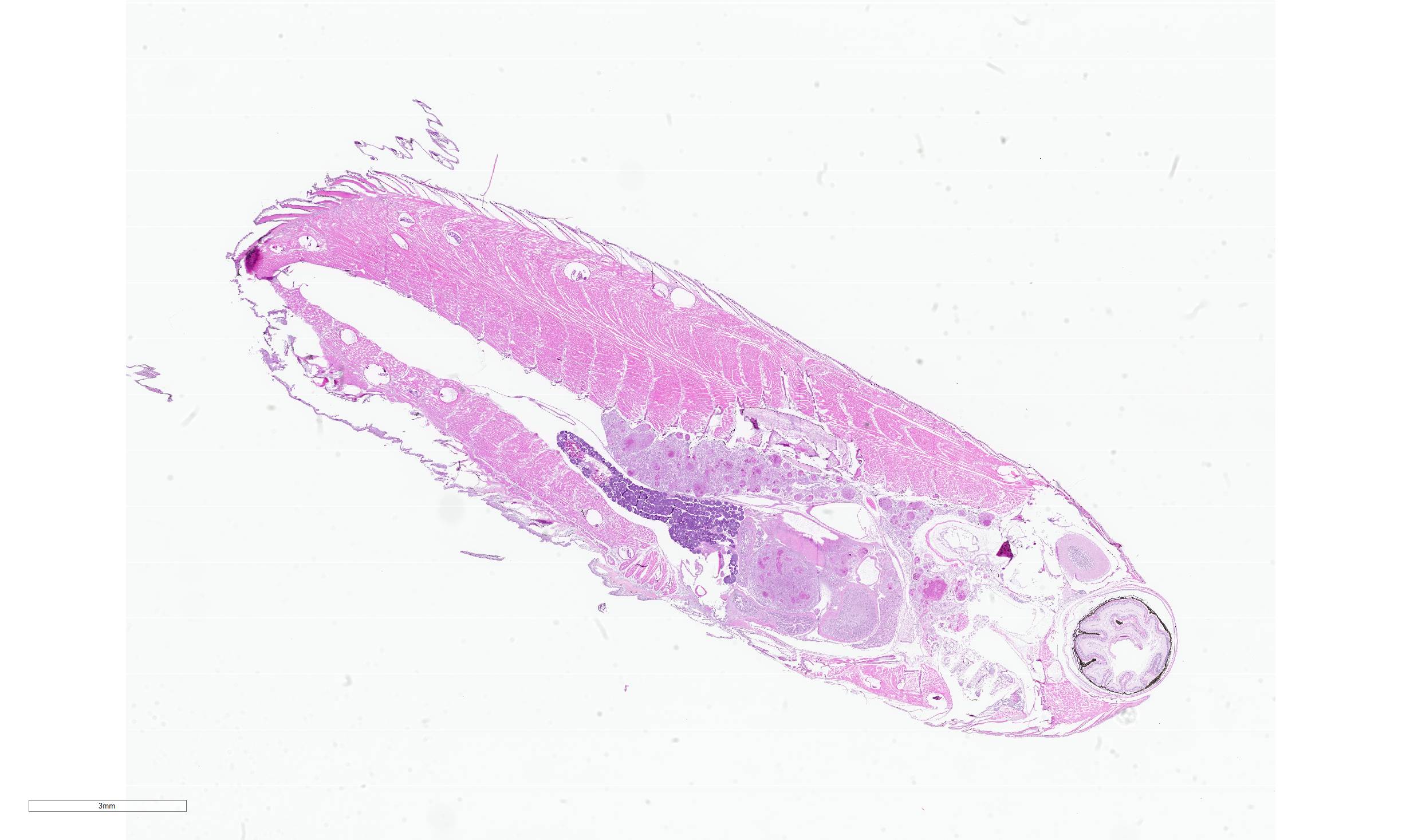
Examined was a 0.25g female betta fish (Betta splendens) in emaciated body condition, evidenced by concavity of the middle abdomen.
Microscopic Description:
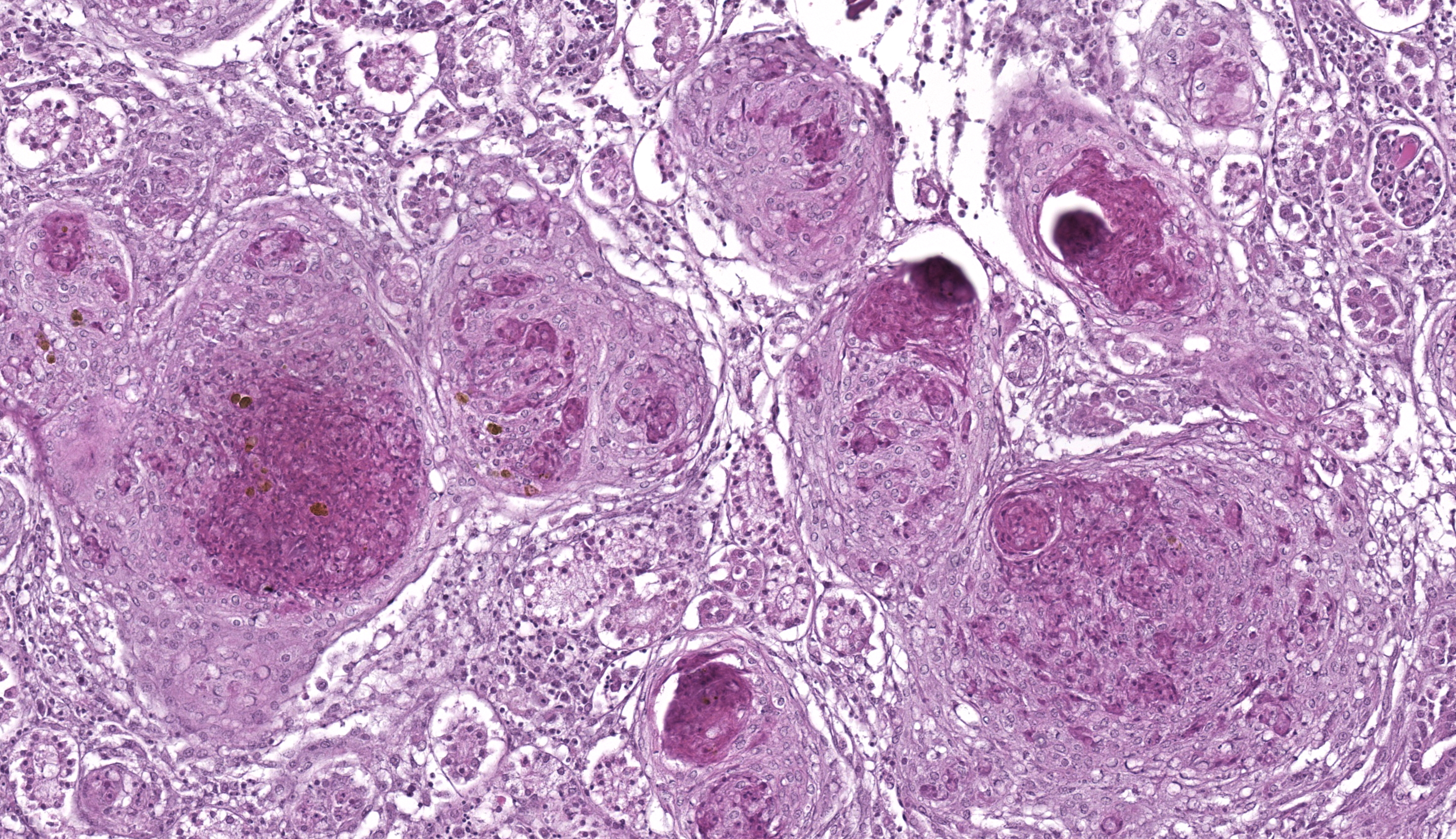
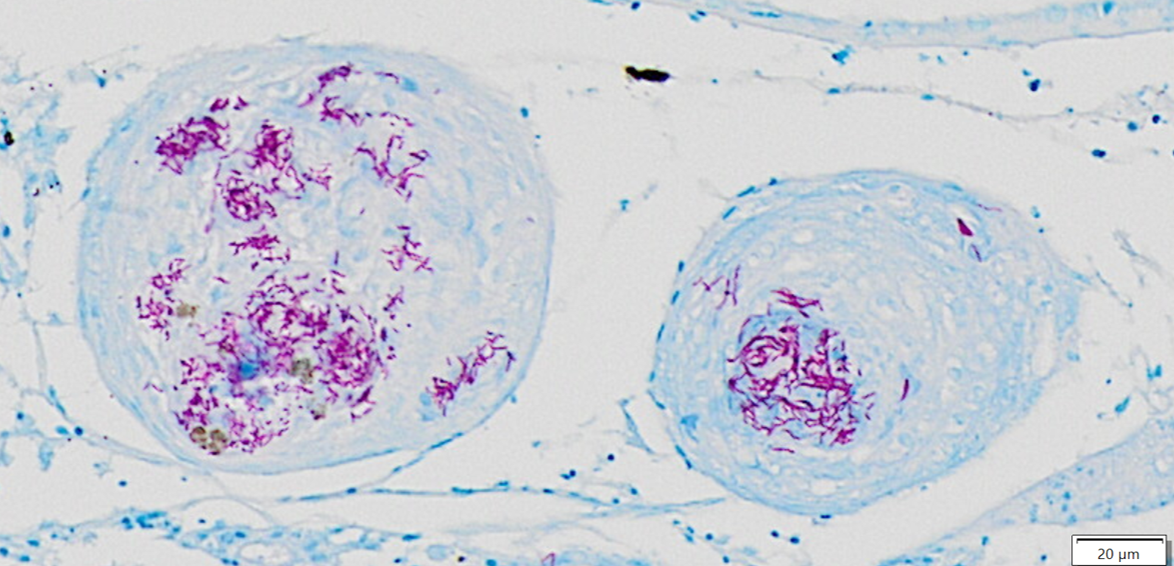
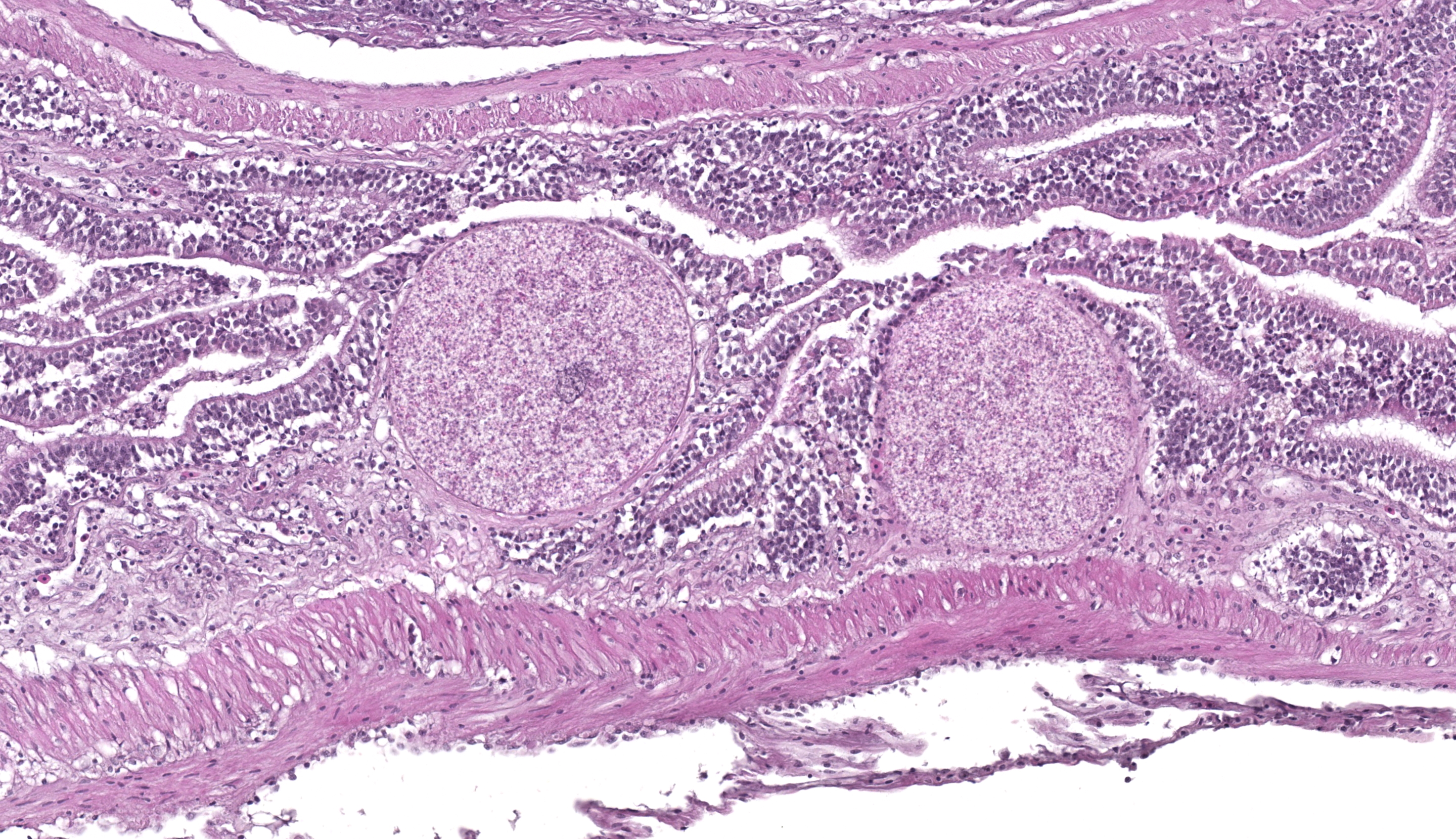
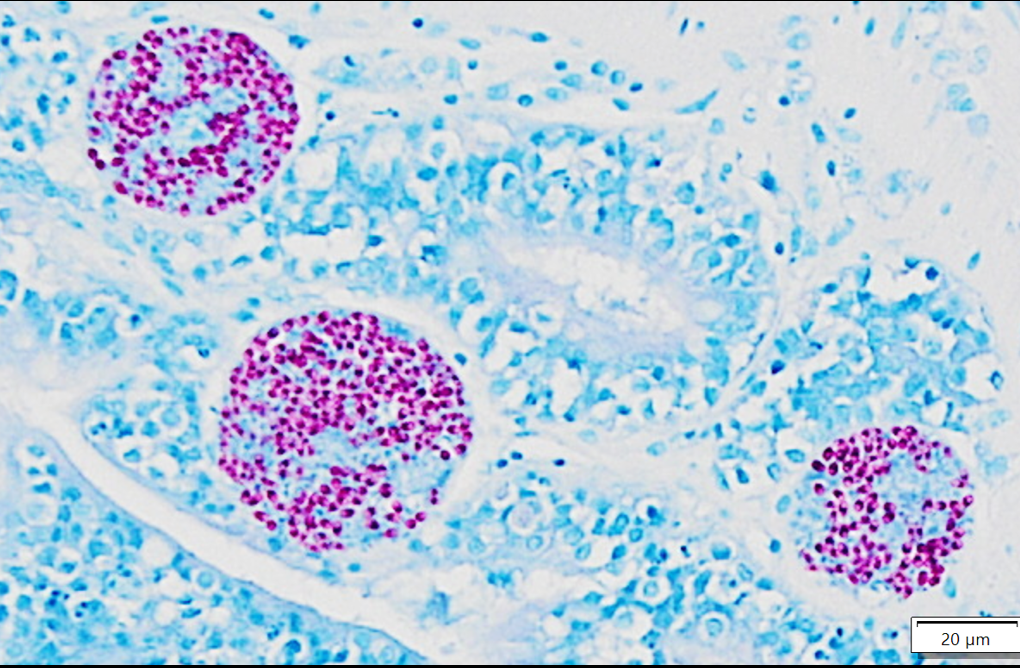
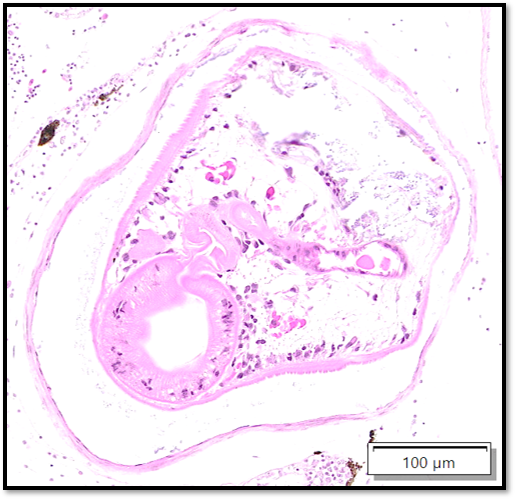
Whole body longitudinal section: Numerous round to oval, 40-400um diameter granulomas were present in the kidneys, liver, intestine, ovary and in the gill chamber. The granulomas were composed of central cores of brightly eosinophilic material admixed with karyorrhectic debris (necrosis) surrounded by concentric layers of epithelioid macrophages. Abundant acid-fast bacterial rods were present within the cytoplasm of the macrophages and in the necrotic cores of the granulomas. The intestinal mucosa contained variably-sized, up to 200um diameter, round microsporidian xenomas. The xenomas had thin eosinophilic walls, contained numerous eosinophilic, 2-4 um, acid-fast oval spores, and some had central hyperplastic host nuclei. Many 0.4-0.6mm diameter round cysts with up to 3um thick eosinophilic hyaline walls were present in the skeletal musculature and body cavity that contained cross sections of trematode larvae. The larvae had 5um thick teguments, lacked body cavities, had abundant parenchymatous matrix, paired ceca, and prominent suckers. Some of the cysts contained only clear space or degenerate cells. Few aggregates of macrophages with intracytoplasmic brown granular pigment (melanomacrophages) sat adjacent to some cysts and within some granulomas.
Contributor’s Morphologic Diagnosis:
- Nephritis, hepatitis, enteritis, branchitis, and oophoritis, multifocal to coalescing, chronic, severe, granulomatous with intralesional acid-fast bacteria
- Trematodiasis, multifocal, skeletal muscle and body cavity
- Microsporidial xenomas, multifocal, intestinal mucosa
Contributor’s Comment:
Mycobacteriosis and nocardiosis are well-recognized diseases of wild and aquacultured fishes in which actinomycetes cause severe chronic granulomatous systemic disease.4 Mycobacteriosis is much more common in fish than nocardiosis, but both produce analogous gross and histologic lesions.10 Bacterial morphology can help to differentiate these, as Mycobacteria spp. are non-filamentous, non-branching while Nocardia spp. are branching filamentous organisms that may resemble Chinese letters.11 Further, the Ziehl-Neelsen acid-fast stain is useful for discrimination as Nocardia spp. stain poorly or not at all with this stain, and are best visualized with modified acid-fast stains, such as Fite’s modified acid-fast stain10. Therefore, the bacteria in this case were consistent with Mycobacteria sp. and bacterial culture or PCR could provide species-level characterization.
Many non-tuberculous Mycobacterium species have been reported to cause disease in fish, although M. marinum, M. chelonea and M. fortuitum are the most common isolates.6 Transmission occurs principally through ingestion of either contaminated feed, organic debris, or infected carcasses.14 Prompt removal of dead or moribund fish within a tank is, therefore, of the utmost importance in preventing transmission. The clinical manifestations of mycobacteriosis in zebrafish are often non-specific and may include emaciation, lethargy, abdominomegaly, and changes in swimming behavior.14 In many cases fish die without exhibiting clinical signs.14 Chronic stress in zebrafish exacerbates the susceptibility to mycobacteriosis and increases the severity of the disease.12 This likely played a role in this case as the fish was transported from a natural environment in Thailand to an experimental setting about 5 weeks prior to death. Notably, aquatic mycobacteria such as M. marinum pose significant zoonotic concerns including cutaneous granulomas and protracted illness, especially in immunocompromised individuals.6
Microsporidia are obligate intracellular eukaryotic parasites that can infect a wide range of hosts.4 Taxonomic classification of microsporidia has shifted over time, and this group is currently considered to be most closely related to fungi based on molecular studies4. Fish are hosts to over 150 species of microsporidia in 14 different genera4. The genera can be divided according to their ability to form xenomas (Glugea, Tetramicra, Loma, Ichthyosporidium, Jirovecia, Microfilum, Microgemma and Nosemoides spp.) or not (Nucleospora, Pleistophora, Heterosporis and Thelohania spp.). Xenomas are cyst-like structures composed of hypertrophied host cells within which reside microsporidians at various life stages. The infected host cell increases in size due to the proliferating parasites and hypertrophy of the infected cell, including the host cell nucleus as seen in this case.
Coinfection of microsporidia with mycobacteria in fish is not uncommon as microsporidosis is more prevalent in immunosuppressed individuals12 and mycobacteriosis suppresses immunity.2 Microsporidial infestation of the gastrointestinal tract when confined to the intestinal mucosa is relatively inconsequential, as was likely in this case, as the surrounding inflammation was minimal.3 Two microsporidians that cause significant disease in laboratory-reared zebrafish are Pseudoloma neurophilia and Pleistophora hyphessobryconis.1 Infection with these can cause subclinical disease or overt illness and may impact experimental outcomes1. Extensive surveillance methods and strict biosecurity measures have been outlined to control or prevent microsporidial infection in zebrafish research facilities.8,13 Further, zebrafish lines that are specific-pathogen free for Pseudoloma neurophilia have been developed.7
The encysted parasites were consistent with metacercariae, the larval form of digenetic trematodes.1 The life cycle of most trematodes involves an aquatic snail intermediate host, in which asexual reproduction occurs leading to the release of cercariae into the water.5 These penetrate the skin or gills of the fish intermediate hosts and often migrate to skeletal muscle for development into encysted metacercariae.5 These are infective to definite hosts after consumption of the fish.5 Natural infection is not associated with clinical disease in zebrafish and the inflammatory response is usually minimal due to the parasitic capsule, but the presence should be noted as infection may impact experimental outcomes.1 Several species of digenean trematodes have metacercarial forms, and common species in freshwater fish in Thailand include Haplorchis pumilo and Centrocestus formosanus.9
Contributing Institution:
Wake Forest School of Medicine
Department of Pathology, Section on Comparative Medicine
Medical Center Boulevard, Winston-Salem, NC 27157
www.wakehealth.edu
JPC Diagnosis:
- Liver, heart, anterior kidney, posterior kidney, coelom, ovary: Granulomatous hepatitis, myocarditis, coelomitis, oophoritis, and nephritis, chronic, multifocal, severe
- Skeletal muscle and coelom: Granulomas, multiple with encysted metazoans
- Intestines: Microsporidial xenomas, multiple
JPC Comment:
This second case showcases several classic fish entities while offering some additional points for consideration. We agreed with the contributor that the multifocal granulomatous inflammation best represented mycobacteria, though we did note small numbers of filamentous, slender bacteria on Fite-Faraco that could reflect Nocardia spp. that could be differentiated via PCR or culture. Within affected tissues, the presence of pigmented macrophages (melanomacrophages) likely reflects sequestration of iron which is a host defense mechanism against mycobacteria. Although termed “melanomacrophages” in many texts, recent studies have demonstrated that these macrophages contain multiple pigments, to include iron and melanin, and may be more appropriately termed “pigmented macrophages.”
The microsporidial xenomas within the intestine were characteristic and easy to identify in this case. Although H&E was sufficient to make the diagnosis, acid-fast stains also sharply outlined spores within affected enterocytes. With the Fite-Faraco stain, morphology was helpful to not confuse a xenoma with adjacent mycobacterial foci given the overlapping distribution of these lesions and staining characteristics. Conference participants also reviewed the anticipated features of non-xenoma forming microsporidial infections which characteristically have wider distribution of spores and associated inflammatory cells instead.
The metazoan parasites of this case gave conference participants pause. The encysted metazoans within skeletal muscle resemble metacercaria, though the metazoans within the coelom appear morphologically distinct and contain 10um round amphophilic to basophilic bodies (possible calcareous corpuscles), consistent with a larval cestode (i.e. a plerocercoid). Tapeworms are a common finding in both free ranging and cultured fish,15 opening up the possibility that this case is in fact a ‘four-fer’.
References:
- Cartner S, Eisen JS, Farmer SF, Guillemin KJ, Kent ML, Sanders GE. The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications. 2019.
- Fenton MJ, Vermeulen MWJI, immunity. Immunopathology of tuberculosis: roles of macrophages and monocytes. 1996;64: 683-690.
- Ferguson HW. Systemic pathology of fish. A text and atlas of comparative tissue responses in diseases of teleosts. Iowa State University Press; 1989.
- Frasca Jr S, Wolf JC, Kinsel MJ, Camus AC, Lombardini ED. Osteichthyes. In: Pathology of wildlife and zoo animals. Elsevier; 2018:953-1001.
- Gardiner C, Poynton SLJ. An atlas of metazoan parasites in animal tissues. 1999.
- Gauthier DT, Rhodes MWJTVJ. Mycobacteriosis in fishes: a review. 2009;180: 33-47.
- Kent ML, Feist SW, Harper C, et al. Recommendations for control of pathogens and infectious diseases in fish research facilities. 2009;149: 240-248.
- Kent ML, Feist SW, Harper C, et al. Recommendations for control of pathogens and infectious diseases in fish research facilities. 2009;149: 240-248.
- Krailas D, Veeravechsukij N, Chuanprasit C, Boonmekam D, Namchote SJAt. Prevalence of fish-borne trematodes of the family Heterophyidae at Pasak Cholasid Reservoir, Thailand. 2016;156: 79-86.
- Lewis S, Chinabut S. Mycobacteriosis and nocardiosis. In: Fish diseases and disorders. Volume 3: viral, bacterial and fungal infections. CABI Wallingford UK; 2011:397-423.
- Mauldin EA, Peters-Kennedy JJJ, Kennedy, 1 PsPoDAV. Integumentary system. 2016: 509.
- Ramsay J, Watral V, Schreck C, Kent MJJoFD. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). 2009;32: 931-941.
- Sanders JL, Watral V, Kent MLJIJ. Microsporidiosis in zebrafish research facilities. 2012;53: 106-113.
- Smith SA. Fish diseases and medicine CRC Press; 2019.
- Scholz T, Kuchta R, Oros M. Tapeworms as pathogens of fish: A review. J Fish Dis. 2021 Dec;44(12):1883-1900.