Wednesday Slide Conference, Conference 17, Case 1
Signalment:
Seven-year-old, male rhesus macaque, Macaca mulatta.
History:
Euthanasia was due to chronic, progressively worsening diarrhea.
Gross Pathology:
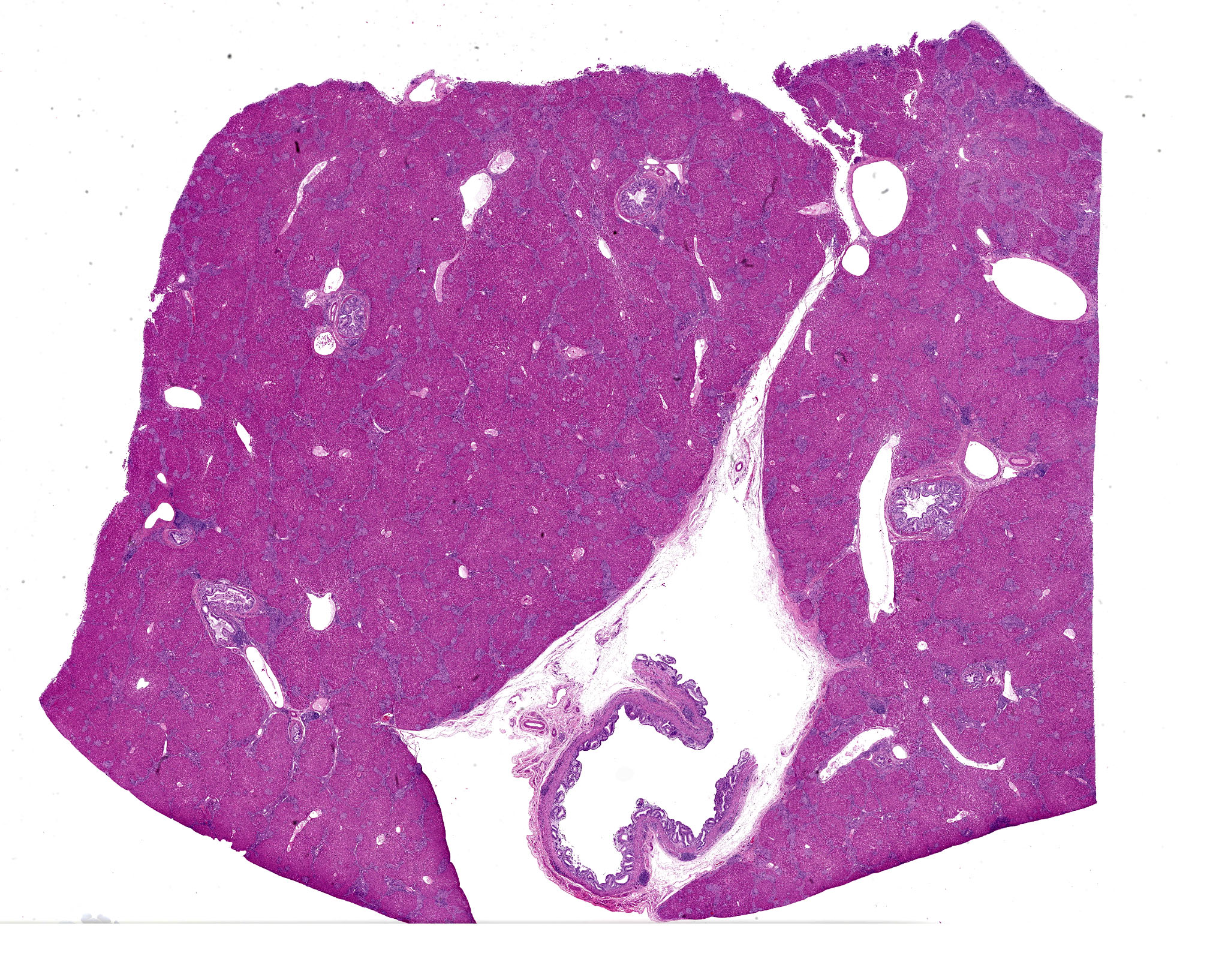
The macaque was in very lean nutritional condition. Liver was firmer than normal, and the common bile duct was dilated and tortuous. Visceral lymph nodes of the abdomen were enlarged, soft and confluent. Large intestines had mucosal thickening.
Laboratory Results:
PCR: 1) Common bile duct: Enterocytozoon bieneusi positive; Cryptosporidium negative.
2) Mesenteric lymph node, spleen and ileum: Mycobacterium negative
Bacteriology: Colon: Colon: E. coli, Klebsiella pneumoniae, Proteus vulgatus, Streptococcus viridans group, & Lactobacillus spp present. Strict Anaerobes: Prevotella oris & Peptoniphilus asaccharolyticus present.
Microscopic Description:
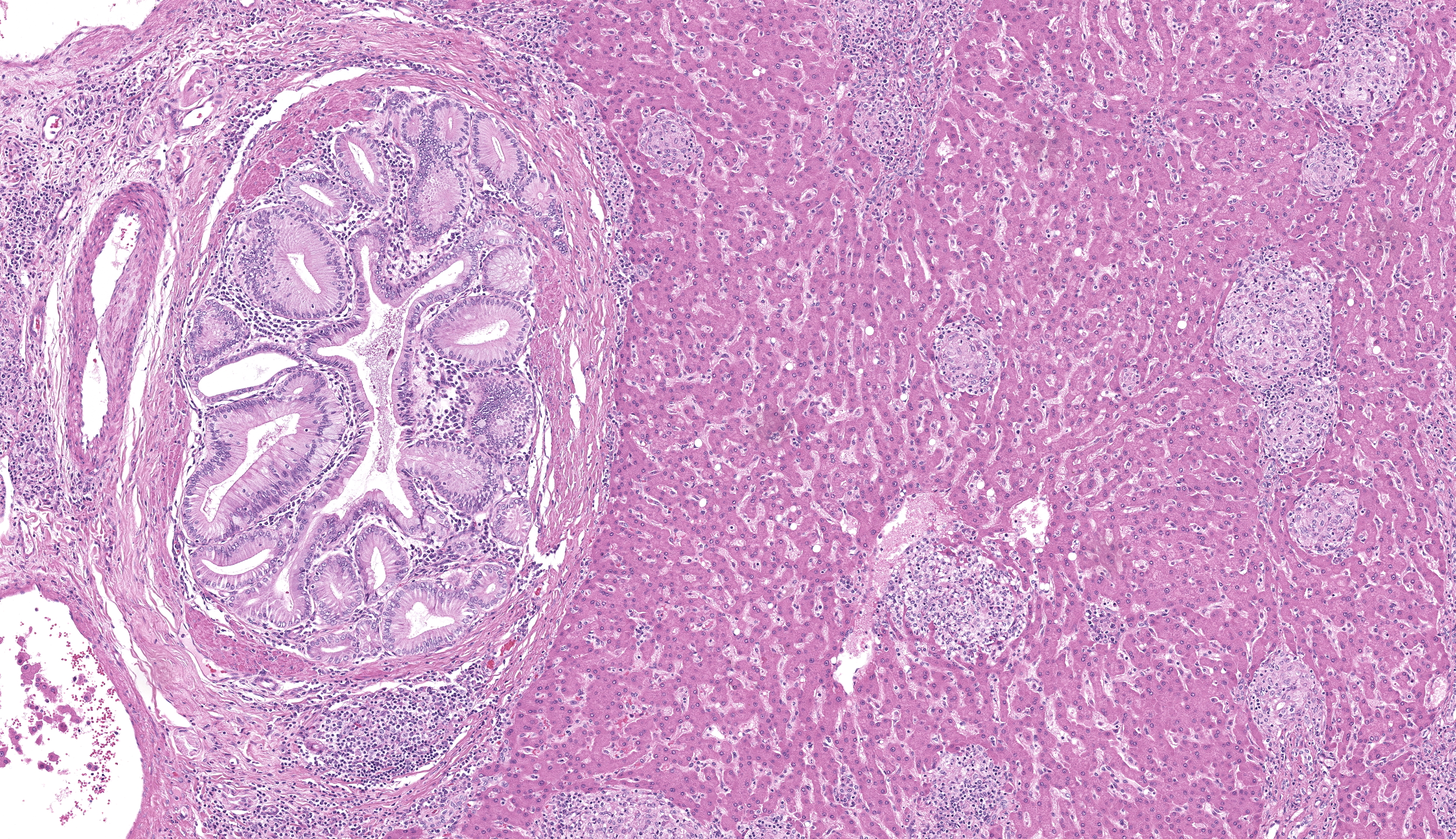
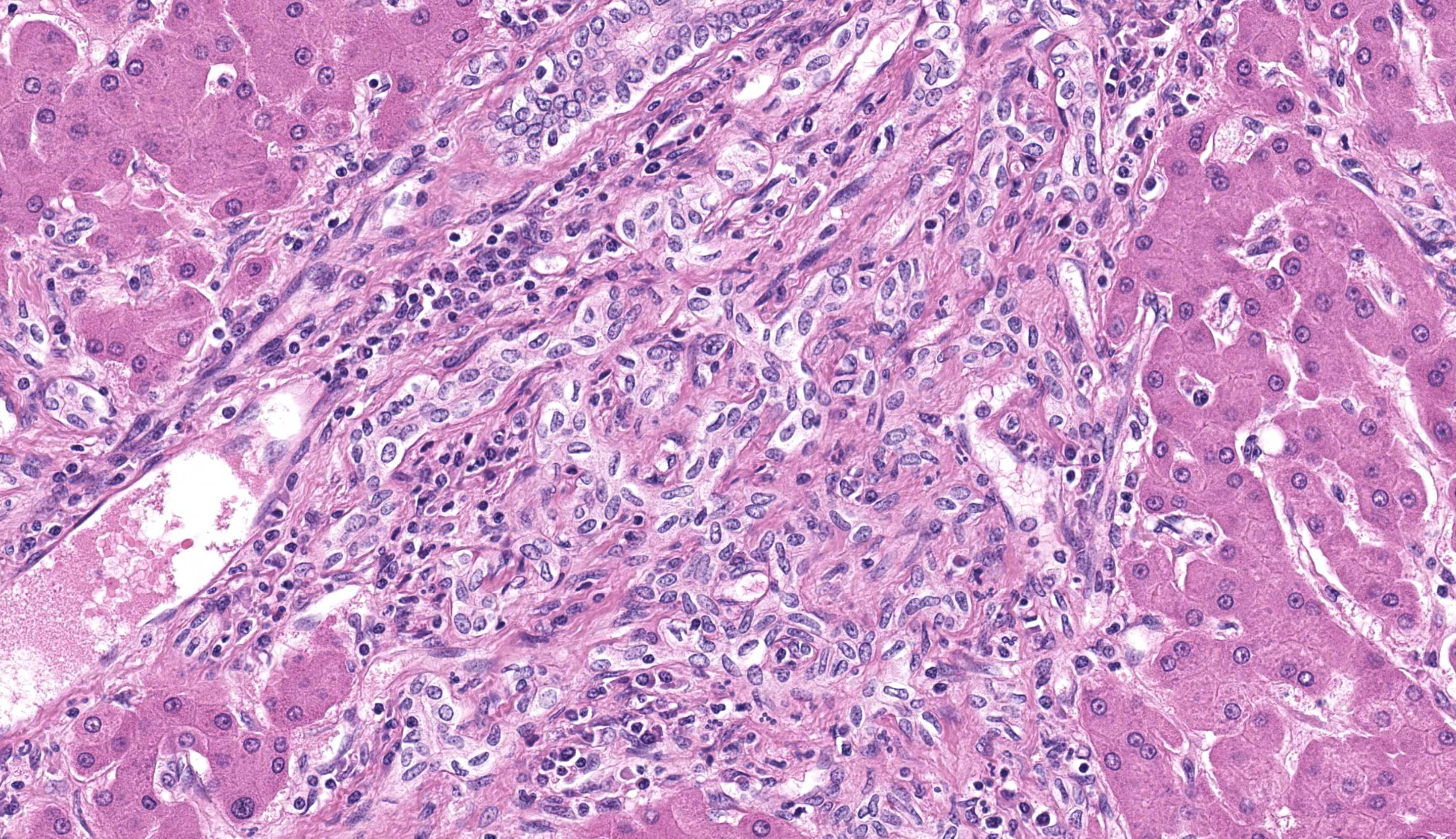
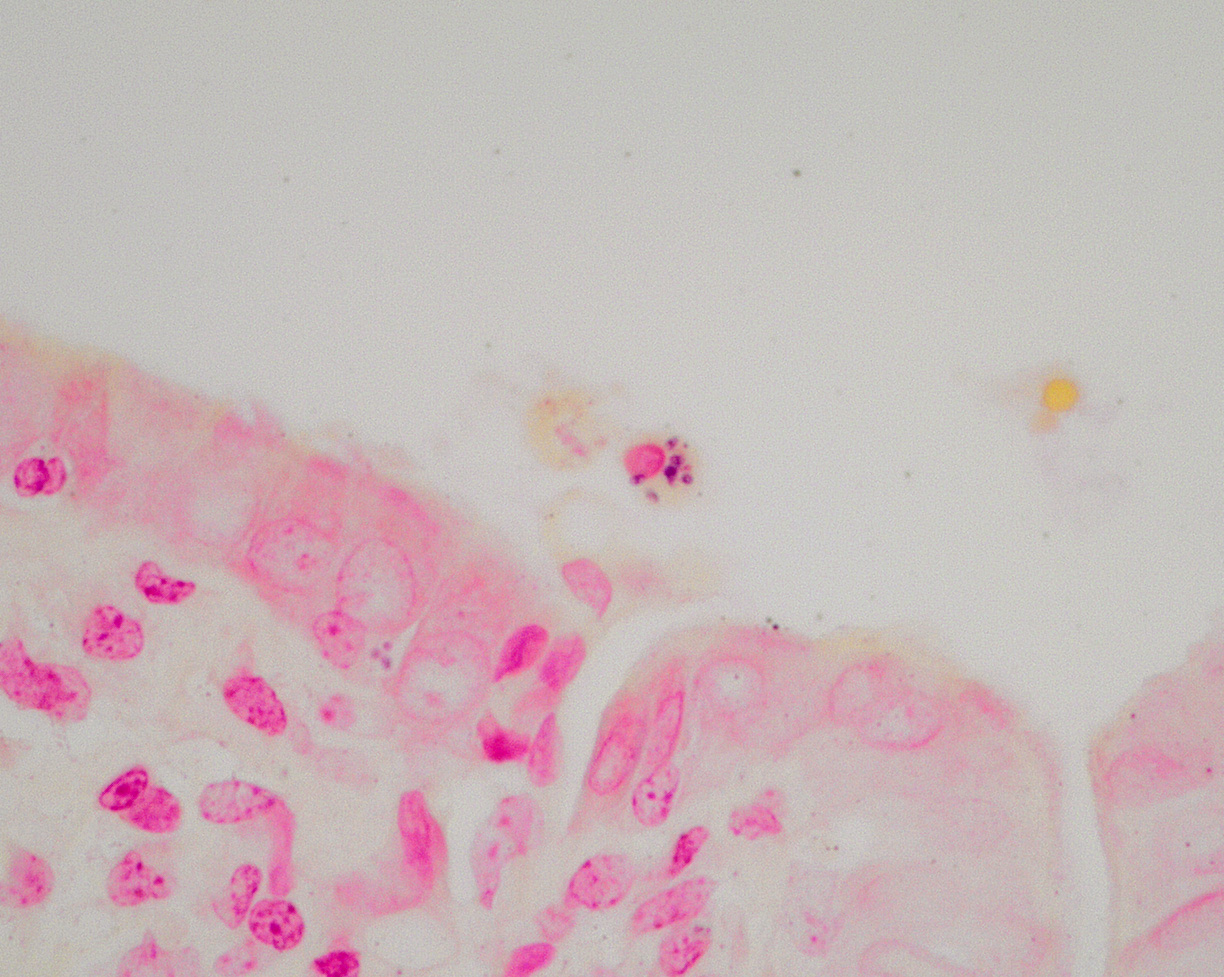
Large intrahepatic bile ducts and the common bile duct had moderate, diffuse mucosal hyperplasia with moderate lymphocytic and plasmacytic inflammation. Portal triads were expanded by similar inflammation and bridging fibrosis is present. Gram stains revealed small colonies of 1-2 µ, gram positive organisms, typically in sloughed biliary epithelium.
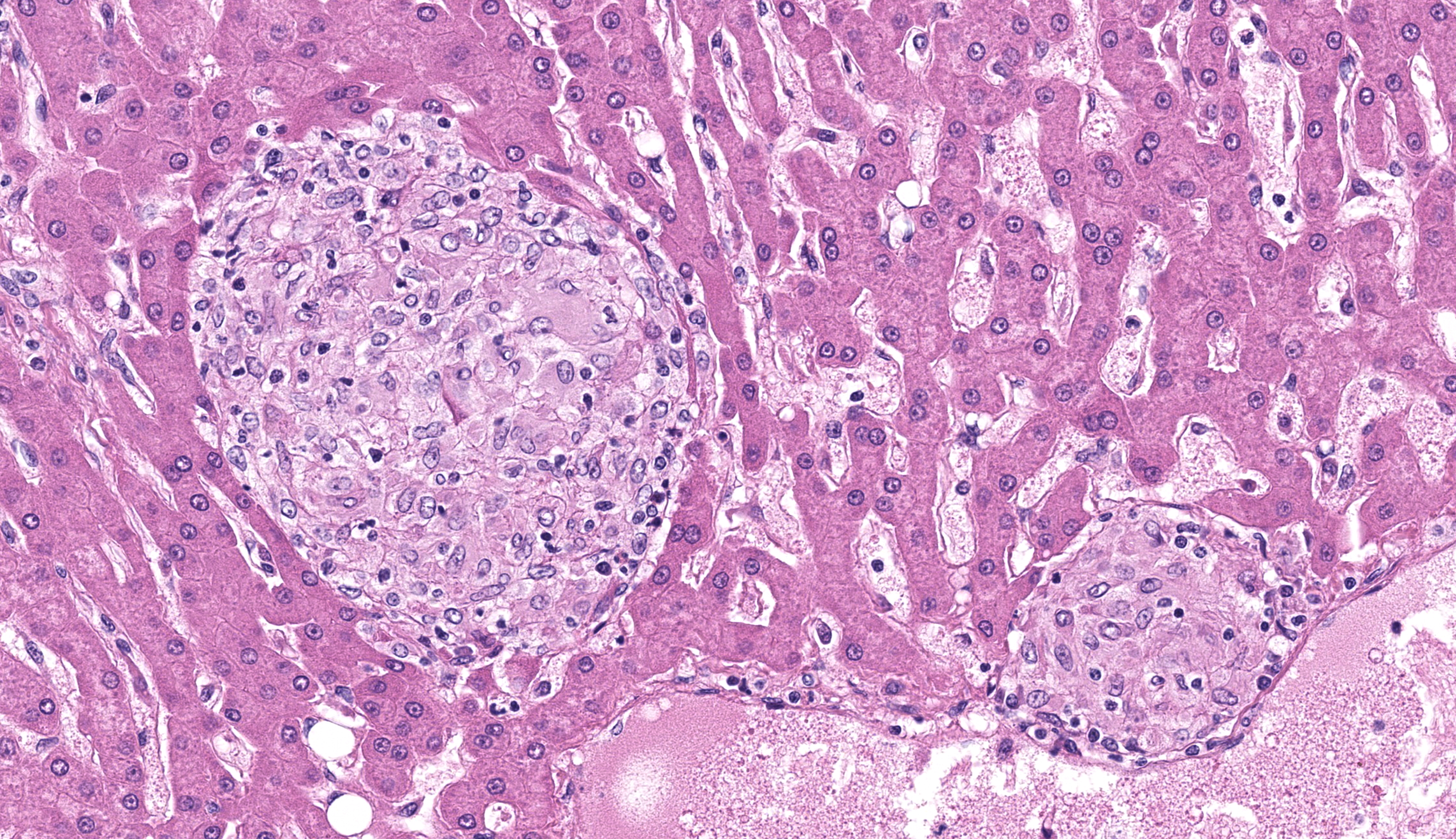
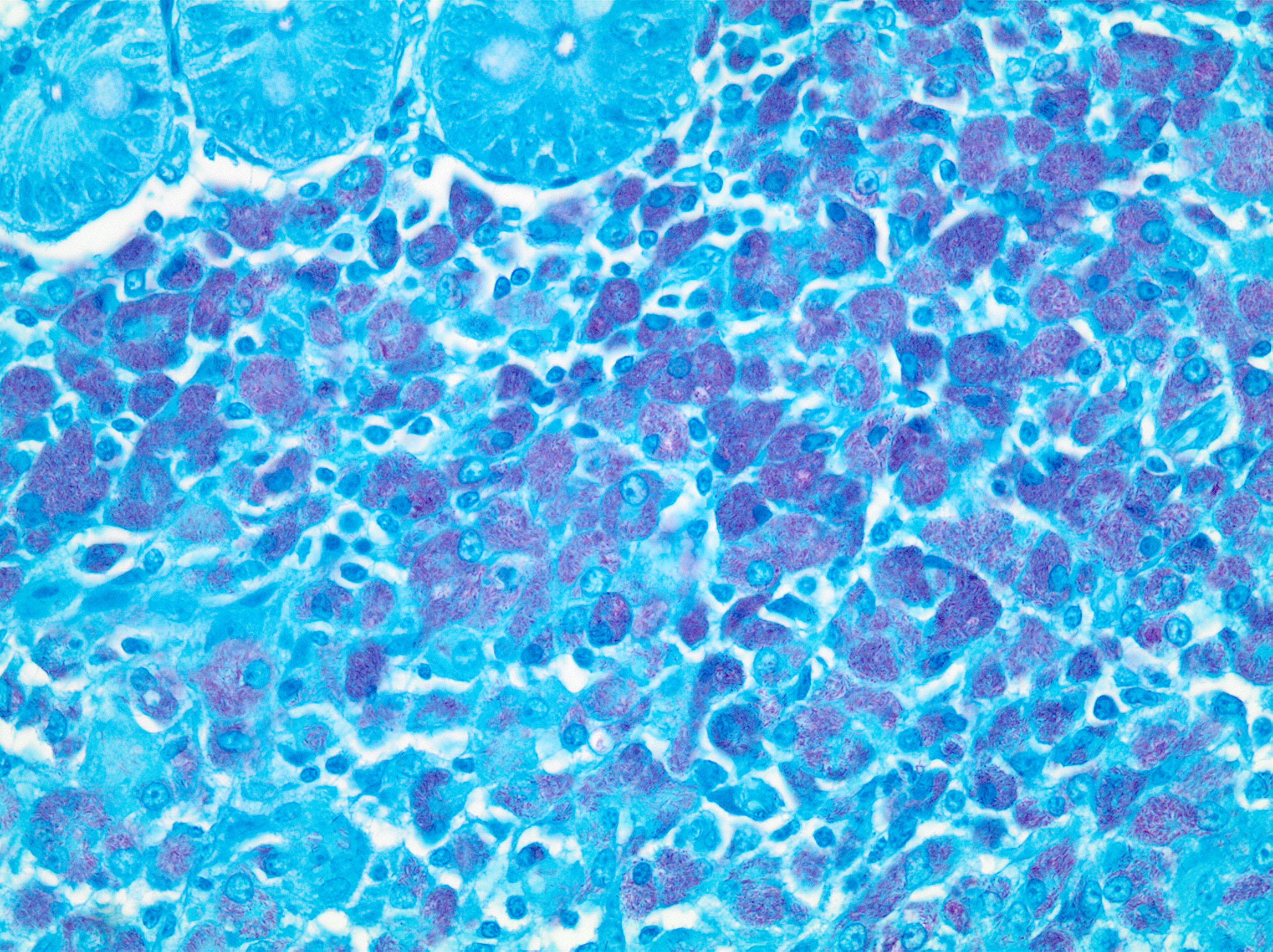
The liver had numerous granulomas in all lobes. Granulomatous inflammation was also present in spleen intestines and axillary and mesenteric lymph nodes. Acid fast stain of liver sections revealed small numbers of acid-fast positive bacilli within granulomas.
The ileum had sheets of epithelioid macrophages with myriad intracytoplasmic acid-fast bacteria.
Contributor’s Morphologic Diagnosis:
Liver: Cholangiohepatitis, hyperplastic, chronic. diffuse, moderate with intracellular protozoa, Enterocytozoon bieneusi.
Liver: Granulomatous hepatitis, moderate, multifocal (Mycobacterium avium complex, presumptive.)
Contributor’s Comment:
Enterocytozoon bieneusi (EB) is a microsporidian parasite that is frequently associated with diarrhea and biliary disease in SHIV and SIV infected macaques. It also is a common cause of chronic diarrhea in human HIV patients. EB isolates are divided into 11 groups and numerous subgroups based on polymorphisms of ribosomal internal transcribed scribed spacer DNA. (ITS).5 Most laboratory primates have organisms from Group 1, subgroup D which is the common strain that affects humans. Wild and zoo apes and monkeys have EB isolated from different groups and/or subgroups. Transmission is likely direct with organisms having been isolated in water and fresh produce. They have also been isolated from mollusks.3,4
EB spores have been isolated from the feces of immunocompetent animals.6 Experimental infection of immune competent macaques by spores of human origin resulted in fecal shedding in about 8 weeks with infections persisting for months, and elevation of ALK but not ALT, GGT, or AST serum liver enzymes. Spores were isolated from bile and feces, but other developmental stages were not found and symptoms were not observed. Histology of the biliary tree of immunocompetent macaques had lymphoplasmacytic cholecystitis and choledochitis.2
Once infected, immunocompromised animals frequently develop symptoms concurrent with a drop in T4 (CD4) lymphocyte counts. If the T4 count remains stable, immunodeficient animals can remain asymptomatic.2 In immune suppressed animals, infection results in hyperplastic cholecystitis and choledochitis with lymphoplasmacytic inflammatory infiltrates. Though EB is associated with diarrhea, coinfections are common so the contribution of EB to clinical signs is often uncertain. All stages of the organism can be identified by in situ hybridization in mucosal cells of the biliary tree, duodenum, and jejunum. Plasmodia are multinucleated; they and sporoblasts measure between 4-12 um. Spores and sporoblasts measuring 0.8-1.5 um can be visualized in sloughed biliary mucosal cells by using either Ziehl-Neelson acid-fast, Brown and Hopps, Weber’s modified trichrome, or methenamine silver stains. Only small numbers of organisms are found this way.2,6,7 Differential diagnoses include another microsporidian organism, Cryptosporidium parvum, and ascending bacterial infections.7
As microsporidia rely on host cells for nucleotides and amino acids, they are considered obligate pathogens.1 Although originally classified as protozoa, they have since been placed in a separate phylum and are thought to closely related to fungi due to protein and structural analysis, and simple sequence repeat RNA (SSR) gene sequencing. They are characterized by chitinous spore walls, coiled polar tubules and a radiolucent vacuole and mitochondria-like organelles that lack mitochondrial genome.1 After ingestion, spores attach to host cell membranes using the polar tubule through which the sporoplasm enters host cell cytoplasm. It then becomes a meront which divides into plasmodia and through sporogony form sporoblasts that mature into spores.3,4 Studies on Encephalitozoon, another microsporidian organism, have found that immunity is depended on CD8 lymphocytes, IFN-gamma and IL-12.3
A commonly associated opportunistic coinfection with EB is atypical mycobacteriosis also referred to a non-tuberculous mycobacterium (NTM). NTM are thought to be acquired from either soil or water. Species of the Mycobacterium avium-intracellulare species complex (MAC) are most frequently identified. Infections are characterized by diffuse granulomatous enterocolitis with large numbers of intracellular bacteria. Disseminated disease also can occur with fewer organisms present in other organs which is the is the pattern of this case. Another common NTM species is M. kansasii which can be present asymptomatically. M genevense, is a species isolated in human HIV patients causes lesions that are similar to MAC with numerous bacteria. One study found that animals were more likely to have symptoms from NTM when coinfections are present.8,10
The reason for the negative mycobacteria PCR results in the presence of numerous bacteria in this case is uncertain. One lab found a 45.1%% sensitivity with PCR compared to culture.9 A source of false negative results with PCR are interfering materials such has immunoglobulin and collagen.11
Contributing Institution:
National Institutes of Health, ORS
JPC Diagnosis:
1. Liver: Cholangiohepatitis, proliferative and lymphoplasmacytic, chronic, diffuse, moderate, with bridging portal fibrosis and intraepithelial microsporidia.
2. Liver: Hepatitis, granulomatous, multifocal, moderate.
JPC Comment:
This week’s moderator is LTC Erica Barkei, JPC’s Chief of Resident Training and Education.
In this section, microsporidia are occasionally visible on H&E and special stains, particularly within sloughed biliary epithelial cells. In this section, mycobacterial “granulomas” do have epithelioid and/or multinucleate giant cell macrophages, but lack surrounding lymphocytes and fibrosis and a central core of necrosis as is typical for Th1 granulomas. As the contributor notes, macrophages containing non-tuberculous mycobacteria typically have fewer cytoplasmic organisms outside of the colon, which may reflect the relatively limited degree of granuloma development within the liver seen in this case.
There are several other important diseases of immunosuppressed macaques to consider (although not in this section.) As the contributor notes, Cryptosporidium may also be seen within the biliary tract, though this causes suppurative inflammation which is noticeably absent in section. Cytomegalovirus (CMV) has characteristic intranuclear viral inclusions and is also necrotizing. Other common con-infections include simian virus 40 (SV40), Candida albicans, Pneumocystis carinii, and Toxoplasma gondii.
References:
- Dean P, Hirt RP, Embley TM. Microsporidia: Why make nucleotides if you can steal them? PLOS Pathogens 2016; 12(11): e1005870.
- Green LC, Didier PJ, Bowers, L C. et al. Natural and experimental infection of immunocompromised rhesus macaques (Macaca mulatta) with the microsporidian Enterocytozoon bieneusi genotype D. Microbes and Infection 6 (2004) 996–1002
- Han B, Weiss LM. Microsporidia: Obligate intracellular pathogens within the fungal kingdom. Microbiol Spectr. Author manuscript available in PMC 2017 October 1
- Li W, Feng Y, Xiao L. Parasite of the month: Enterocytozoon. Trends in Parasitology, 2021, 20(20):1-2
- Li W, Feng Y, Santin M. Host Specificity of Enterocytozoon bieneusi and public health implications. Trends in Parasitology. 2019, 35(6):436-451
- Mansfield K, Carville A, Herbert D, et al. Localization of persistent Enterocytozoon bieneusi Infection in normal rhesus macaques (Macaca mulatta) to the hepatobiliary tree. J Clinical Microbiol. 1998, 36(8): 2336–2338
- Mansfield K, Carville A, Shevetz D, et al. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in Simian-Immunodeficiency-Virus-Inoculated macaques with hepatobiliary disease. Am J of Path.1997,150(4): 1395-1405
- Maslow J, Brar I, Smith G, et al. Latent infection as a source of disseminated disease caused by organisms of the Mycobacterium avium Complex in Simian Immunodeficiency Virus–Infected Rhesus Macaques. Journal of Infect Dis. 2003; 187:1748–55
- Park JS, Choi J, Lim, J, et al. The combination of real-time PCR and HPLC for the identification of non-tuberculous mycobacteria. Ann Lab Med. 2013, 33:349-352
- Procop G. HIV and mycobacteria. Seminars in Diagnostic Pathology, 34(2017) 332-339.
- Sidstedt M, Rådström P, Hedman J. PCR inhibition in qPCR, dPCR and MPS mechanisms and solutions. Analytical and Bioanalytical Chem. 2020, 412:2009–2023