Signalment:
3-month-old, male, castrated, Finn x Dorset lamb (
Ovis aries).On October 6, the animal was sedated with ketamine and dexmedetomidine and maintained with
isoflurane anesthesia to debride a preputial laceration (presumed shearing injury). The animal was treated with
intramuscular enrofloxacin once daily and flunixin meglumine twice daily until October 9, when the animal was
noted to be passing pink urine (positive for blood and protein by dipstick). Diminished body condition was noted at
this time. Flunixin was discontinued, and animal was started on parenteral ceftiofur and daily subcutaneous fluids.
Pink urine was again noted on October 11. Due to declining clinical course, the animal was euthanatized on October
12.
Gross Description:
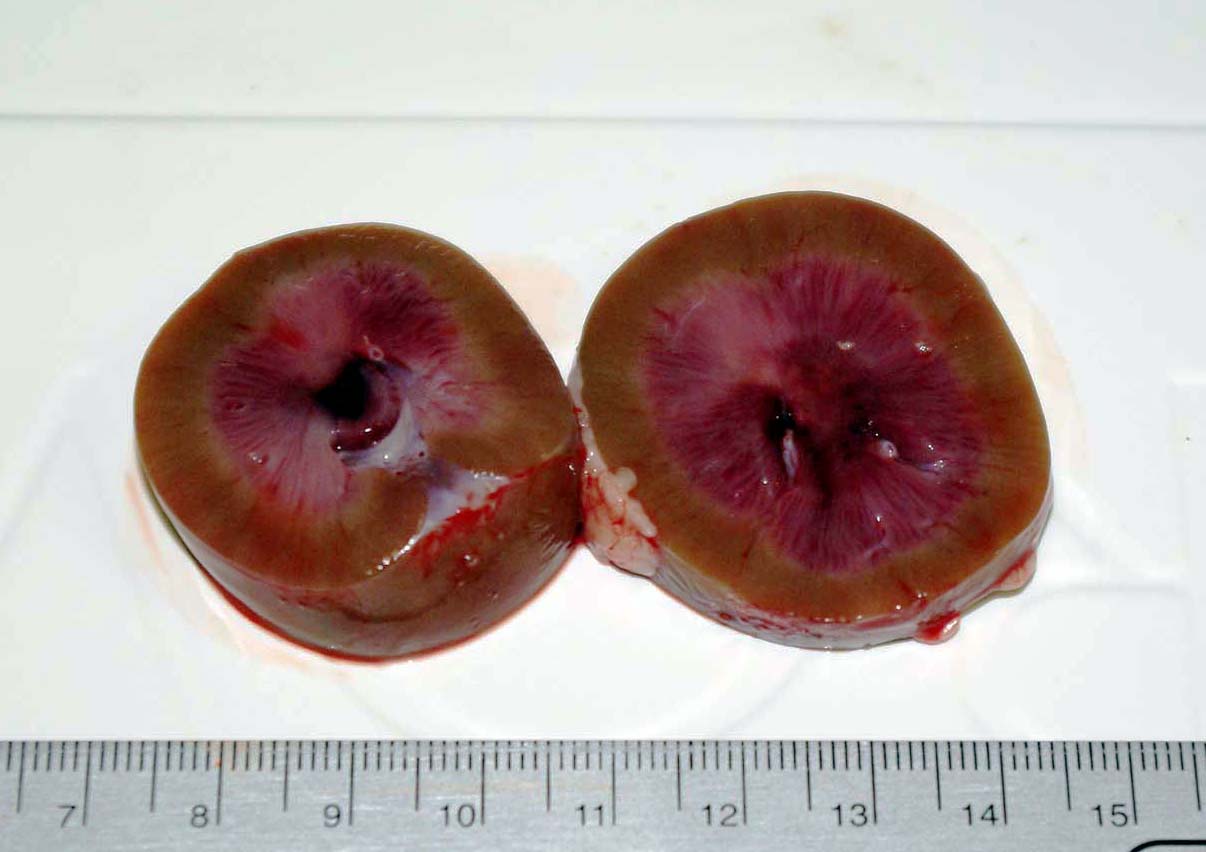
There was mild muscle wasting, although visceral fat reserves were adequate. In both the left
and right kidneys there were focally extensive and severe hemorrhages in the renal medulla and crest with pallor of
the medulla (figure 1). The renal cortices were grossly normal.
Histopathologic Description:
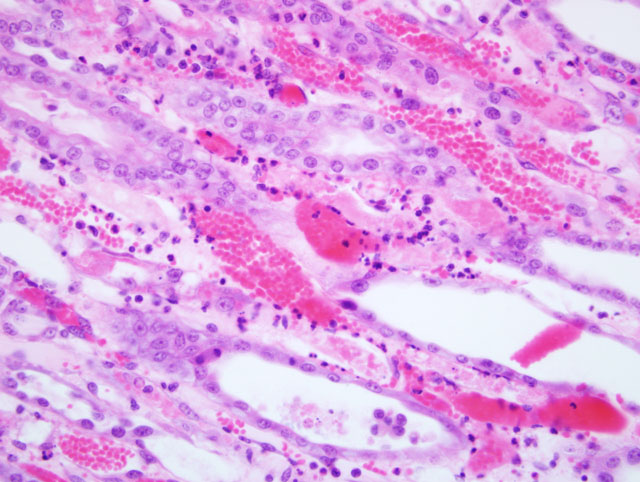
Kidney: Sections submitted include renal medulla, crest and pelvis with variable
amounts of cortex (taken from both kidneys). Within the deep medulla and crest there is focally extensive and
severe subacute coagulative necrosis of tubules and interstitium with edema. Surrounding this is a zone of tubular
epithelial degeneration and necrosis. Epithelial regeneration in this zone is intense, with intact basement membranes
and interstitium. Numerous tubules contain luminal casts of erythrocytes or necrotic cellular debris. Low to
moderate numbers of viable and occasionally degenerate neutrophils are present within small vessels as well as the
interstitial matrix. The cortex is largely unaffected, although in a few sections there are remote, non-occlusive,
adherent fibrin thromboemboli in cortical radial veins. A small amount of proteinaceous exudate is present within
the uriniferous space of some glomeruli.
Morphologic Diagnosis:
Kidneys, bilateral, medulla and crest, necrosis, focally extensive,
subacute, severe, with tubular erythrocyte casts and epithelial regeneration.
Lab Results:
(clinical pathology, microbiology, PCR, ELISA, etc.):
CBC:
  WBC 17,100 x 103/μL (4,000-12,000)
    Neutrophils 10,431 x 103/μL (61%; 400-6000)
    Lymphocytes 6156 x 103/μL (36%; 1,600-9,000)
    Monocytes 171 x 103/μL (1%; 0-750)
    Eosinophils 342 x 103/μL (2%; 0-1200)
  HCT 34% (27-45)
  Hb 10.8 g/dL (9-15)
  Adequate platelets
  Fibrinogen 640 mg/dL
Serum Chemistry:
  Na 146 mmol/L (139-152)
  K 5.3 mmol/L (3.9-5.4)
  Cl 87 mmol/L (95-103)
  Tbili 0.4 mg/dL (0.14-0.32)
  Creatinine 3.8 mg/dL (1.0-2.7)
  BUN 86 mg/dL (8-20)
  Protein 5.2 g/dL (6.0-7.9)
  Albumin 3.1 g/dL (2.4-3.0)
  CPK 273 U/L (42-62)
  LDH 481 U/L (83.1-475.6)
Urinalysis: Clear, straw colored, SG 1.008, pH 8.5, protein 3+, glucose negative, ketones negative, small blood,
WBC 0-1/HPF, RBC 10-20/HPF.
Aerobic blood cultures obtained from the jugular vein were negative for bacterial growth.
Condition:
NSAID toxicity
Contributor Comment:
Clinical signs and gross and histologic lesions are consistent with ischemic necrosis of
the renal medulla and crest due to non-steroidal anti-inflammatory drugs (NSAIDs).(3,6) Anatomically, this lesion is
appropriately termed renal medullary crest necrosis in sheep and horses, and renal papillary necrosis in rodents,
man, and dogs.(7) Prostanoids are produced by cyclooxygenase-1 (COX-1) and COX-2 in the kidneys and exert a
number of autocrine and paracrine effects. Most significantly, the prostanoids prostaglandin E2 and PgI2 modulate
renal blood flow and glomerular filtration rate.(6) Additional COX products play roles in renal handling of sodium
and release of renin. Renal distribution of the COX-1 and particularly COX-2 isoforms and susceptibility to NSAID
nephrotoxicity is species-dependent.(2) The medulla and papilla/crest predominantly express the COX-1 isoform in
most species, where prostanoids products modulate urine concentrating ability, antagonize vasopressin-mediated
water and solute reabsorption, alter distal tubule potassium secretion, and promote dilation of the vasa recta to
maintain medullary blood flow. Rats and dogs also express COX-2 in the papillary interstitial cells, and are
relatively sensitive to developing renal papillary necrosis,(2) suggesting that NSAIDs may also be directly toxic to the
interstitial cells.3 Humans are relatively resistant to NSAID nephropathy, and there is typically intercurrent disease
or overdose (analgesic abuse).(2) Flunixin meglumine is a potent and non-specific COX inhibitor. Recently, the
NSAID diclofenac has been associated with vulture population declines in southern Asia as a result of relay
toxicosis and acute renal failure.(4)
In addition to NSAIDs, renal papillary necrosis can be induced by tyrosine kinase inhibitors, and can be observed
spontaneously in diabetes mellitus, sickle cell disease, and pyelonephritis.(5) Papillary necrosis has also been
documented in dehydrated racing greyhounds and with amyloidosis in cats.(3) Renal papillary antigen-1 has been
proposed as a urinary biomarker of papillary necrosis.(5)
Although fluoroquinolones and cephalosporins can be associated with renal toxicity, this classically presents as an
acute interstitial nephritis (AIN) with mononuclear and occasionally eosinophilic infiltrates suggestive of a
hypersensitivity reaction.(1) Certain members of both classes of drug have also been associated with tubular crystal
development.
JPC Diagnosis:
1. Kidney, medulla: Tubular degeneration, necrosis and regeneration, diffuse, moderate to severe,
with few cellular casts.
2. Kidney, medulla: Coagulative necrosis, acute, focally extensive (infarct).
Conference Comment:
As indicated by the contributor, based on the clinical history the tubular changes are most
likely due to NSAID administration. Participants reviewed the mechanisms of toxicity elegantly outlined by the
contributor. Conference participants interpreted the acute renal crest infarct as most likely a perimortem event
resulting from thrombosis of vessels.
In general, the differential diagnosis for renal papillary/crest necrosis across species includes hypoxic and toxic
insults (which may occur in concert), dehydration, and urinary obstruction with or without pyelonephritis.(3) The
contributor provides an excellent explanation of the pathogenesis of toxic insults. Dehydration contributes to
papillary necrosis in dogs (primarily racing greyhounds) and lambs and kids treated with phenothiazines.(3) Urinary
obstruction and pyelonephritis was high on the differential list for some participants due to the observation of
moderate numbers of neutrophils within the renal crest. However, in this case most of the neutrophils occur in the
medullary interstitium, and not in the tubules. The moderator emphasized that ascending pyelonephritis from the
lower urinary tract typically produces a neutrophilic tubulitis, which is not evident in the case of this animal.
References:
1. John R, Herzenberg AM. Renal toxicity of therapeutic drugs.Â
J Clin Pathol. 2009;62:505-515.
2. Khan KN, Venturini CM, Bunch RT, et al. Interspecies differences in renal localization of cyclooxygenase
isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity.Â
Toxicol Pathol.
1998;26:612-620.
3. Maxie MG, Newman SJ. Urinary system. In: In: Maxie MG, ed.Â
Jubb, Kennedy and Palmers Pathology of
Domestic Animals. 5th ed., vol. 3. Philadelphia, PA: Elsevier Ltd; 2007:425-522.
4. Oaks JL, Gilbert M, Virani MZ, et al. Diclofenac residues as the cause of vulture population decline in Pakistan.
Nature. 2004;427:630-633.
5. Price SA, Davies D, Rowlinson R, et al. Characterization of renal papillary antigen 1 (RPA-1), a biomarker of
renal papillary necrosis.Â
Toxicol Pathol. 2010;38:346-358.
6. Radi ZA. Pathophysiology of cyclooxygenase inhibition in animal models.Â
Toxicol Pathol. 2009;37:34-46.
7. Read WK. Renal medullary crest necrosis associated with phenylbutazone therapy in horses.Â
Vet Pathol.
1983;20:662-669.