CONFERENCE 25, CASE 4
Signalment:
Adult, female intact, North American beaver (Castor canadensis)
History:
The beaver was found in a creek bed and animal control found the beaver lethargic and unresponsive. Following capture, the animal was reported to be “rolling in the crate”. The beaver was brought to a local wildlife rehabilitation center where she was reported to be neurologic and ataxic while walking with impaired vision. Euthanasia was elected and the animal submitted for necropsy exam.
Gross Pathology:
A 7.14 kg intact female beaver (Castor canadensis) was submitted for necropsy, 3 days postmortem and in fair postmortem condition. The nutritional state was fair based on small amounts of visceral and subcutaneous adipose tissue, and muscling was adequate. The lungs, heart, liver, kidneys, spleen, and gastrointestinal tract were unremarkable. The stomach contained a moderate amount of pale tan and fibrous digesta. The small intestine contains a moderate amount of tan to grey, mucoid digesta. The large intestine and rectum contain an abundant amount of well-formed feces.
Laboratory Results:
Rabies antigen and highly pathogenic avian influenza PCR of fresh brain was negative. Canine Distemper IHC had variable immunoreactivity in the nucleus of neurons and astrocytes in the brain (interpreted as artifact) and negative by PCR on formalin fixed, paraffin embedded brain and lung.
Microscopic Description:
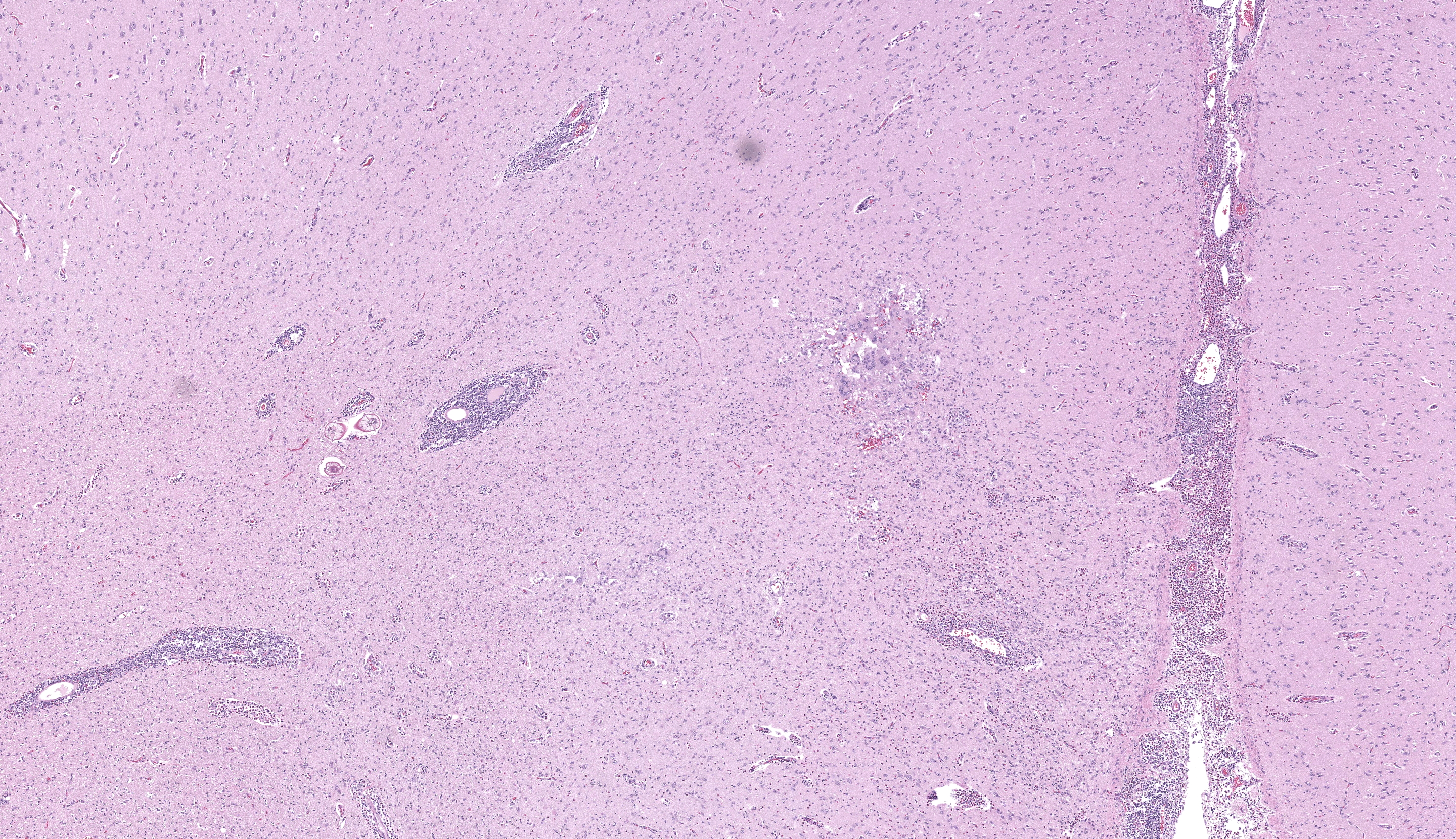
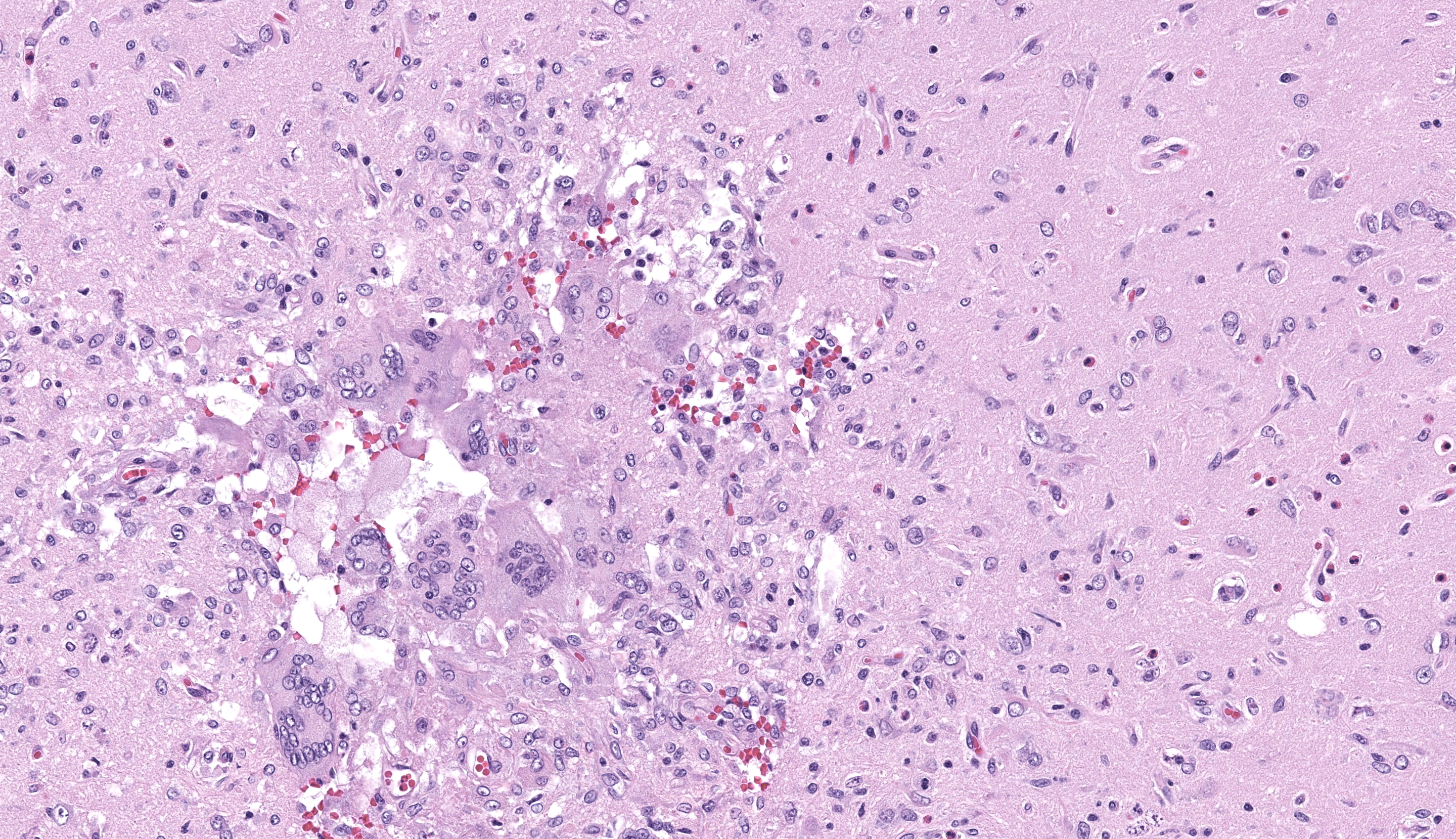
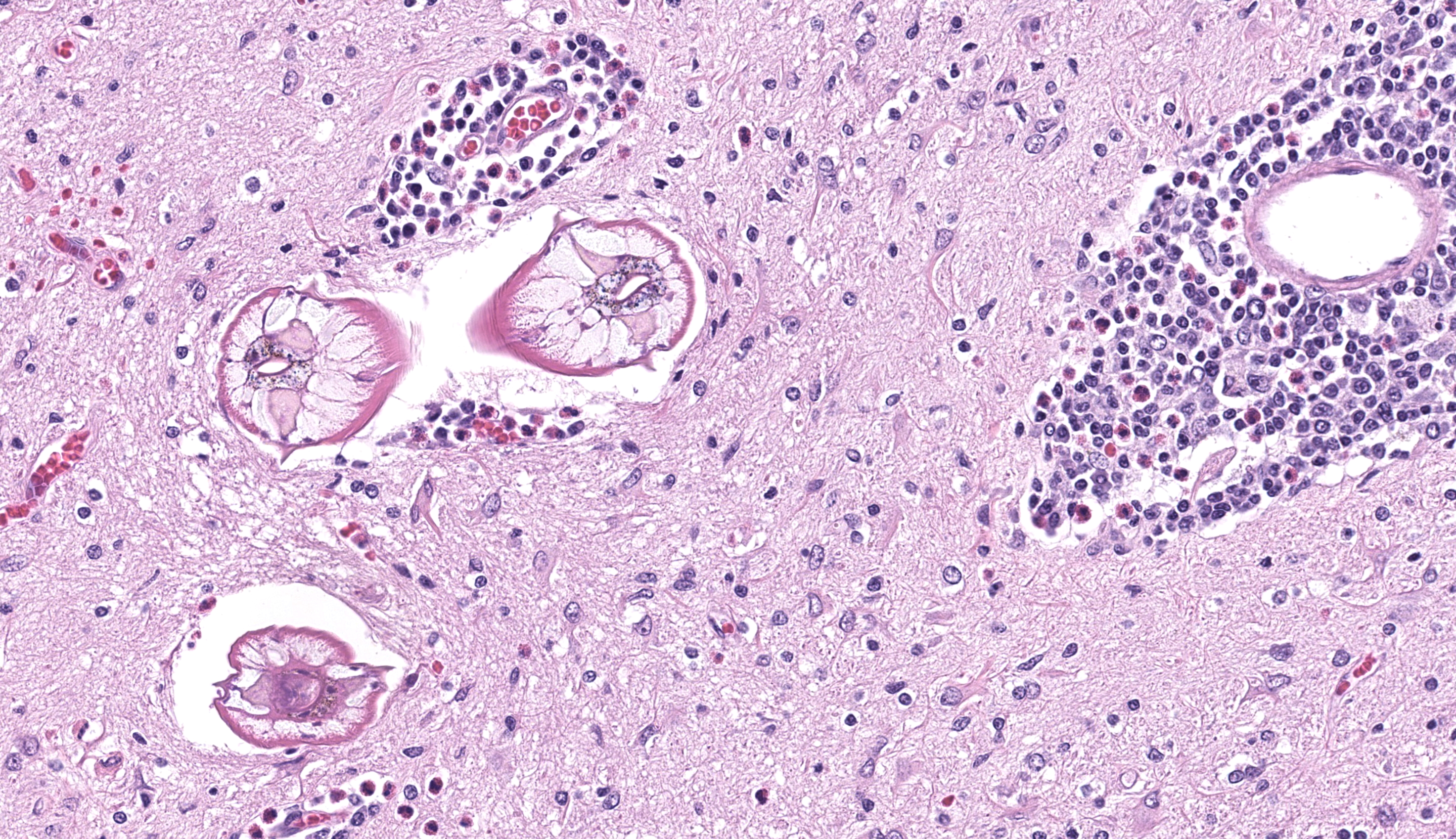
Brain, cerebrum: Examined are sections of cerebrum, hippocampus, and mid-brain. Predominately affecting the left hemisphere of the cerebrum, numerous eosinophils, macrophages, lymphocytes and plasma cells expand the perivascular and meningeal space. Abundant eosinophils and fewer plasma cells, and increased glial cells are scattered throughout the neuropil of affected areas. Embedded within the neuropil of the white matter are cross and tangential sections of larval nematodes. The nematodes are approximately 70 µm in diameter with a 2.5 um thick cuticle and prominent lateral chords and lateral alae. The pseudocoelom contains coelomyarian musculature and digestive tract composed of uninucleate columnar cells. Scant inflammatory cells surround the nematodes. Multifocally within the grey and white matter are foci of necrosis characterized by disruption, loss and vacuolation of the neuropil and presence of macrophages and Langerhans type multinucleated giant cells with fewer lymphocytes and glial cells, and scant plasma cells. Throughout the cerebrum, there are rare necrotic neurons and neurons surrounded by glial cells (satellitosis). The white matter of the midbrain is rarified with bands of eosinophilic, fibrillar material, increased clear space, glial cells and astrocytes. Rare neurons within the adjacent gray matter are variably replaced by homogenous to coarsely stippled basophilic mineral.
Brain, brain stem: Section contains histologically unremarkable brain stem.
Brain, pituitary, pars distalis: Section contains histologically unremarkable pars distalis.
Contributor’s Morphologic Diagnosis:
Brain, cerebrum: Eosinophilic and necrogranulomatous meningoencephalitis, chronic, severe, with gliosis and nematode larvae
Brain, midbrain: White matter degeneration, chronic, moderate, with astrogliosis and astrocytosis (glial scar)
Contributor’s Comment: Baylisascaris species are nematodes that share the order Ascaridia with Toxocara canis and Toxocara cati. The most widespread and ubiquitous Baylisascaris is B. procyonis. Raccoons (Procyon lotor) and dogs (Canis familiaris) are the definitive hosts of B. procyonis, although numerous species of mammals and birds have been identified as intermediate hosts,3 including a report in two American beavers (Castor canadensis).2 In raccoons, B. procyonis is confined to the intestines and rarely causes disease.3 In definitive hosts, eggs hatch and develop into adults in the intestine and subsequently females lay eggs that are passed into the environment with feces, continuing the cycle. Consumption of contaminated feces or an infected host may cause reinfection in the definitive host or infection in intermediate hosts. In intermediate hosts, larvae do not develop into adults, but can migrate to numerous tissues such as the brain (neural larval migrans, NLM), eye (ocular larval migrans, OLM), and viscera (visceral larval migrans, VLM) where they may cause substantial disease and death.3 The precise pathogenesis of larval migrans is unclear, however, migration through the intestinal epithelium into the portal vasculature or lungs has been proposed.7
Compared to Toxocara spp., B. procyonis is particularly pathogenic due to aggressive tissue migration,6,9 continued growth and development of larval migrans,6,9 and release of tissue damaging proteins and host eosinophilic toxins.4,6,8. Most often, lesions are nodular foci consisting of larvae encased in granulomas with substantial eosinophilic inflammation. In this case, there was substantial meningoencephalitis and there were granulomas, but larvae were not observed encased in a granuloma (perhaps as a function of cut).It has been reported that encapsulation of larva takes longer in the central nervous system.7
B. procyonis is reported to be widespread in raccoons in North America with a prevalence between 37 and 82% of raccoons affected.6,11 B. procyonis has also spread through Europe with the introduction of American raccoons.12 B. procyonis is recognized as zoonotic and it can cause fatal visceral and neural larval migrans in children, though ocular migrans is more common in adults.3 In a survey of 150 adults from California, as many as 7% of non-symptomatic adults tested seropositive, suggesting that the prevalence in human populations may be higher than previously thought.13
At least nine species of Baylisascaris have been described (Table 1), with the most notable being Baylisascaris procyonis due to its destructive larval migration. It is also the only known species of Baylisascaris to infect humans. While reports of larval migrans in Baylisascaris procyonis are common, in other species confirmed disease in naturally infected paratenic hosts are rare (Table 1). However, all species of Baylisascaris are potentially pathogenic, as most species have been shown to experimentally infect and cause disease in rodents.10
In this case, the only other histologic findings were a mild eosinophilic and lymphoplasmacytic enterocolitis with a single, intraluminal, unidentified nematode egg in cross section and a focal lymphoplasmacytic interstitial nephritis with minimal eosinophils. Parasites are common in wildlife species, and the eosinophilic enterocolitis and intraluminal parasitic egg is consistent with a parasitic enteropathy. The clinical significance of these findings is unknown.
Table 1. Baylisascaris species and definitive hosts with reported larval migrans in natural infected paratenic hosts.
|
Baylisascaris spp. |
Definitive host(s) |
Natural Paratenic Host |
|
Baylisascaris columnaris |
Skunks |
Primates15 |
|
Baylisascaris devosi |
Fishers, martens, wolverines |
No reports |
|
Baylisascaris laevis |
Marmots, ground squirrels |
No reports |
|
Baylisascaris melis |
Badgers |
No reports |
|
Baylisascaris potosis |
Kinkajou |
No reports |
|
Baylisascaris procyonis |
Raccoon, dogs |
Humans,14 mammals, and birds1 |
|
Baylisascaris schroederi |
Giant Panda |
No reports |
|
Baylisascaris transfuga |
Bears |
Moose5 |
|
Baylisascaris venezuelensis |
Spectacled bear |
No reports |
Contributing Institution:
Colorado State University
Department of Microbiology, Immunology, and Pathology
https://vetmedbiosci.colostate.edu/vdl/
JPC Morphologic Diagnosis: Transverse cerebrum at the level of the thalamus: Meningoencephalitis, necrotizing, eosinophilic, and granulomatous, subacute, multifocal and asymmetric, marked, with gliosis and larval ascarids.
JPC Comment: The final case of this conference provides both overlapping and differing features in comparison to the P. tenuis meningoencephalitis observed in Case 2 in a sitatunga. Cross-sections of larval Baylisascaris were focal in the sections we reviewed, though the expansion of the leptomeninges and reactive astrocytes with distinct processes are a solid indication of a more clinically severe process that led to the decline of this animal. While both cases were necrotizing, there is a sizable eosinophil contingent as well as the presence of multinucleated giant cell macrophages which are conspicuous here. We considered the chronicity of this case at length – did the presence of multinucleated giant cells not indicate a transition to chronic inflammation? The presence of inflammatory cells within the neuroparenchyma (reminder from Case 1 discussion: the number of eosinophils in the brain is should still be zero) is an indicator of the breach of the blood-brain barrier, and the entry of large histiocytes should theoretically take longer given their size and circulating number. Dr. Koehler stood fast on subacute chronicity, noting that the large numbers of eosinophils and macrophages can appear relatively quickly within the brain (within 72 hours) and that meningeal fibrosis was a better arbiter of the actual transition to chronic inflammation – this was not a feature of this case at all. Conversely, the large numbers of intact eosinophils argued against a longer time course as lifespan within tissue is several days on average and overall numbers should decline with time. Accordingly, the features of this case certainly straddle the line of acute and chronic, though the clinical history of ‘Justin Beaver’ (this animal’s actual given name) leaves much information to our imagination and allows both camps to feel vindicated in their interpretation. Finally, we extend a special thank you to the contributor for their excellent summary (Table 1) of Baylisascaris across species and hope others will find it useful.
References:
- Bauer C. Baylisascariosis--infections of animals and humans with 'unusual' roundworms. Vet Parasitol. 2013;193: 404-412.
- Desprez I, Yabsley MJ, Fogelson SB, et al. Baylisascaris procyonis larva migrans in two captive north american beavers (Castor canadensis). J Zoo Wildl Med. 2017;48: 232-236.
- Gavin PJ, Kazacos KR, Shulman ST. Baylisascariasis. Clin Microbiol Rev. 2005;18: 703-718.
- Hamann KJ, Kephart GM, Kazacos KR, Gleich GJ. Immunofluorescent localization of eosinophil granule major basic protein in fatal human cases of Baylisascaris procyonis infection. Am J Trop Med Hyg. 1989;40: 291-297.
- Hoberg EP, Burek-Huntington K, Beckmen K, Camp LE, Nadler SA. Transuterine infection by Baylisascaris transfuga: Neurological migration and fatal debilitation in sibling moose calves (Alces alces gigas) from Alaska. Int J Parasitol Parasites Wildl. 2018;7: 280-288.
- Kazacos KR. Baylisascaris procyonis and related species. In: W. M. Samuel MJP, and A. A. Kocan, eds. Parasitic diseases of wild mammals. 2 ed. Ames, IA: Iowa State University Press; 2001:301-341.
- Kazacos KR. Visceral, ocular, and neural larva migrans. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, eds. Pathology of infectious diseases. Stamford, CT: Appleton and Lange; 1997:1459-1473.
- Moertel CL, Kazacos KR, Butterfield JH, Kita H, Watterson J, Gleich GJ. Eosinophil-associated inflammation and elaboration of eosinophil-derived proteins in 2 children with raccoon roundworm (Baylisascaris procyonis) encephalitis. Pediatrics. 2001;108: E93.
- Richardson D, Krause P. North American Parasitic Zoonoses, vol. 6. Norwell, MA: Kluwer Academic Publishers; 2003.
- Sapp SGH, Gupta P, Martin MK, et al. Beyond the raccoon roundworm: The natural history of non-raccoon Baylisascaris species in the New World. Int J Parasitol Parasites Wildl. 2017;6: 85-99.
- Straif-Bourgeois S, Cloherty E, Balsamo G, Gee L, Riegel C. Prevalence of Baylisascaris procyonis in Raccoons Trapped in New Orleans, Louisiana, 2014-2017.
Vector Borne Zoonotic Dis. 2020;20: 22-26. - Umhang G, Frantz AC, Ferté H, et al. Surveys on Baylisascaris procyonis in two of the three French wild raccoon populations. Int J Parasitol Parasites Wildl. 2024;23: 100928.
- Weinstein SB, Lake CM, Chastain HM, et al. Seroprevalence of Baylisascaris procyonis Infection among Humans, Santa Barbara County, California, USA, 2014-2016. Emerg Infect Dis. 2017;23: 1397-1399.
- Wise ME, Sorvillo FJ, Shafir SC, Ash LR, Berlin OG. Severe and fatal central nervous system disease in humans caused by Baylisascaris procyonis, the common roundworm of raccoons: a review of current literature. Microbes and Infection. 2005;7: 317-323.
- Zimmerman D, Dangoudoubiyam S, Kazacos K. Serological diagnosis of Baylisascaris procyonis in primates using a human ELISA test. Journal of Wildlife Medicine. 2019;50: 414-420.