Wednesday Slide Conference, Conference 1, Case 1
Signalment:
Aborted thoroughbred equine foetus (Equus caballus).
History:
A late gestation aborted thoroughbred foetus was submitted for postmortem examination. No placenta was submitted. According to the submitted clinical history, the mare had been vaccinated for equine herpesvirus and had no history of previous abortions.
Gross Pathology:
The equine foetus was presented dead, in a fair state of preservation. Fetal weight was 40 kg and body length 110 cm (estimated gestational age between 300 and 330 days). No placenta was available for examination. There were no gross lesions on external examination. Examination of the thoracic cavity revealed diffusely firm and rubbery lung lobes (non-aerated). No other lesions present.
Microscopic Description:
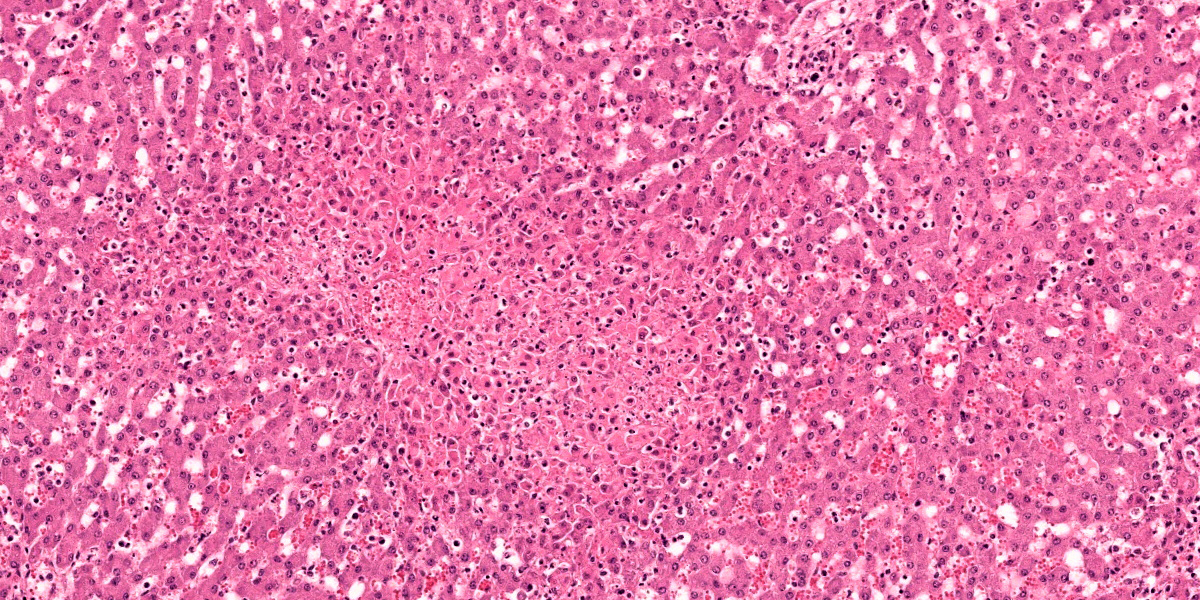
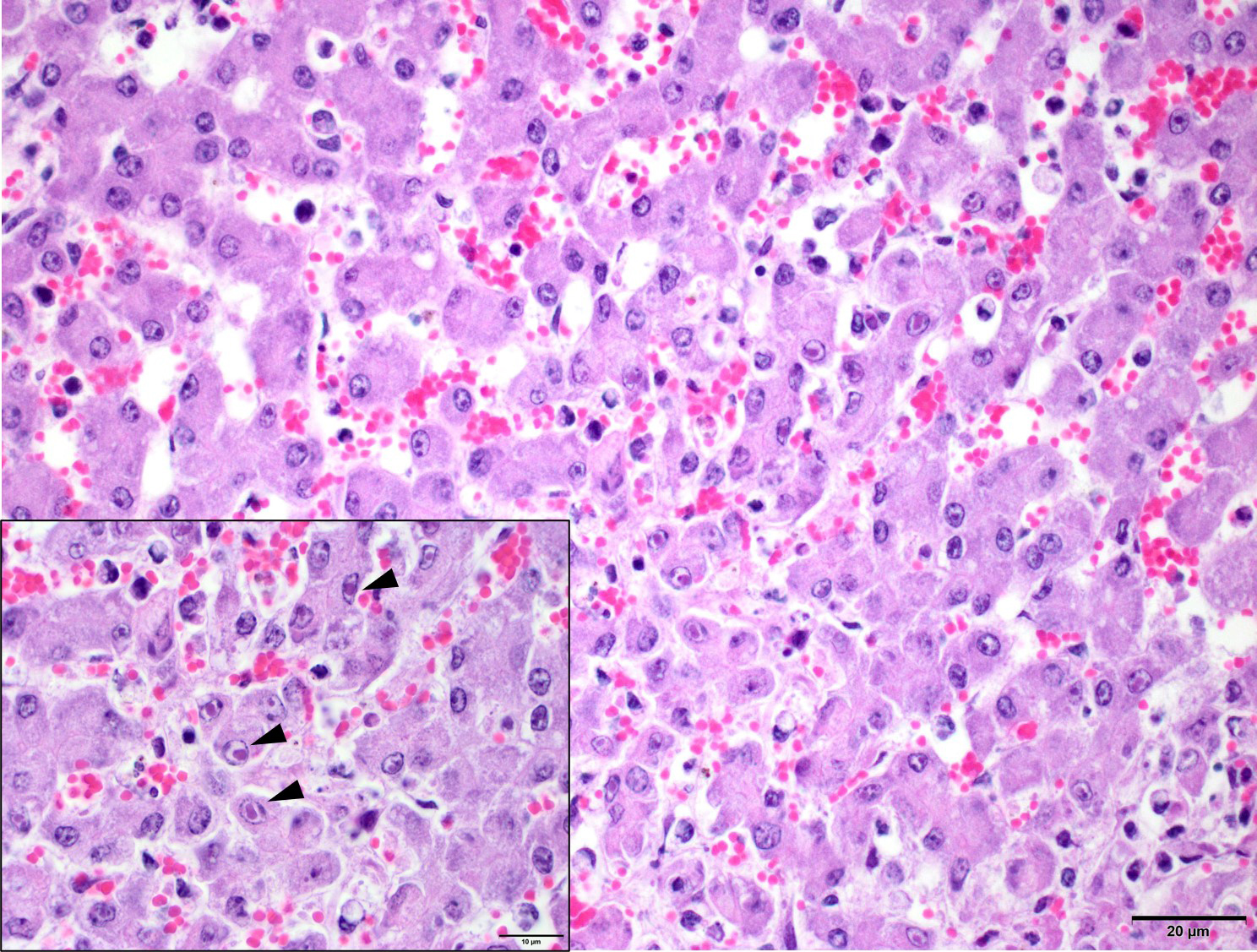
Liver: Within the hepatic parenchyma are multifocal, random areas of hepatocellular necrosis characterised by sinusoidal disruption, accumulation of karyorrhectic cellular debris, hepatocyte hypereosinophilia, cellular swelling, nuclear pyknosis and rare mixed inflammatory cells (Fig.1). Hepatocytes in adjacent areas frequently contain 2-4 um, pale eosinophilic intranuclear inclusions that displace the nuclear chromatin to the periphery (Fig. 2).
Hepatic sinusoids are mildly congested. Periportal areas are moderately expanded by oedema and moderate numbers of lymphocytes, plasma cells and macrophages. Occasionally, small to medium calibre blood vessel walls are expanded by oedema, and necrosis of endothelial cells, characterised by pyknosis, cellular swelling, karyorrhexis and karyolysis.
Contributor’s Morphologic Diagnosis:
Liver: Hepatocellular necrosis, acute, multifocal, random, with hepatocellular intranuclear inclusion bodies.
Contributor’s Comment:
Equine herpesvirus type-1 (EHV-1) and equine herpesvirus type-4 (EHV-4) are important ubiquitous pathogens of all breeds of horses and other equids worldwide. In general, EHV-1 strains are associated with respiratory disease, abortion, and paresis/paralysis, whereas EHV-4 strains are predominantly associated with respiratory disease.2-4
In New Zealand, and similar to the rest of the world, most cases of equine herpesviral abortions are due to EHV-1. Equine herpesvirus type-1 (EHV-1) is a large, enveloped, DNA virus that is classified within the family Alphaherpesviridae in the order Herpesvirales. The virus is spread via multiple routes, including fomites, fetuses and placentas from EHV-1 induced abortions, and particularly by horse-horse contact and contamination. Horses shed EHV-1 in their respiratory secretions for up to three weeks post-infection.3 Once infection is established, subclinical latency in the trigeminal ganglion occurs, and helps viral transmission between horses, which may or may not be associated with signs of illness.3,5
In clinically ill animals, following exposure, the virus replicates in a restricted plaque wise manner in the epithelial cells lining the upper respiratory tract,5,6 including the pharynx, nasal turbinates, soft palate, and tracheal epithelium.4 Entrance into epithelial cells is established either via direct fusion with the plasma membrane, or by endocytosis and fusion with an endosomal membrane.3 After infecting the respiratory tract, the virus crosses the basement membrane via infected leukocytes (CD172+ cells and T/B lymphocytes).5 Once through the basement membrane, these infected leukocytes, penetrate connective tissues and enter the bloodstream and draining lymph nodes within 24-48 hours post infection.4,6 Thus, viraemia via infected leukocytes disseminates the virus around the body, resulting in pyrexia and lymphadenomegaly.5 Once in the circulation, EHV-1 can travel around the body and establish infection in target organs, including the pregnant uterus or the central nervous system.3,5 This is established via strong adherence of EHV-1 infected leukocytes to endothelial cells, which subsequently results in an inflammatory environment that recruits additional monocytes to the endothelium.5 The resulting inflammatory cascade may promote further endothelial cell infection, via increased expression of adhesion molecules (selectins secondary to release of IL-2 and TNF-alpha) on endothelial cells and stronger adherence of infected leukocytes.7 After adherence, the virus replicates in the endothelial cells of the uterus, allantochorion, and umbilical vein.8 The resulting vasculitis and thrombosis, damages the placenta and disrupts blood supply, leading to abortion6. This has been demonstrated in experimentally infected animals, where a lymphocytic vasculitis, focal thrombosis, and infarction of the microcotyledons results in abortion.8 Abortion typically occurs, after 2-12 weeks in late gestation, and the incubation period varies widely from 9-120 days. 4,8 Interestingly, most cases result in no obvious uterine lesions, however in the presented case a severe metritis was diagnosed in the mare. However, it has been reported that there are no long term effects on reproductive performance in affected mares.2
As previously mentioned, viral latency can also develop, this occurs after replication in the respiratory epithelium. The virus enters nerve endings of the peripheral nervous system, including the trigeminal ganglia, and sympathetic and parasympathetic neurons that innervate the respiratory epithelium.5 Here it travels via retrograde transport to the sensory and autonomic peripheral ganglia.5 Along with this, it has been demonstrated to establish latency in the circulating T lymphocytes. Under stress, reactivation of EHV-1 replication occurs and particles spread from these infected T lymphocytes or trigeminal neurons through anterograde transport to the respiratory epithelium.5
Gross foetal lesions described in the literature include icterus, splenomegaly, perirenal oedema, pulmonary oedema or haemorrhage,
and pale miliary foci on the surface of the liver.8,9 In the present case, no gross lesions were present in the foetus, and a definitive cause for the abortion was not established during gross postmortem examination.
Confirmation of EHV-1 infection can be done via a number of ways, including immunohistochemistry of frozen or paraffin embedded tissues, and PCR for direct and rapid detection in frozen or paraffin embedded tissues.8 Additionally, histological lesions, if present, can be used to support a diagnosis of EHV-1. Respiratory lesions include uniform pulmonary interlobular septal oedema and infiltration with mononuclear inflammatory cells, with fibrinous alveolar exudation and necrosis of bronchial and alveolar epithelial cells.7 Hepatic lesions are not as common, but include focal necrosis, oedema, and leukocytes in the necrotic foci and portal triads. The acidophilic inclusion bodies in the nuclei of hepatic parenchymal cells surrounding these necrotic foci are specific for EHV.6,7
Preventative measures include management strategies and vaccination of breeding mares. Separating animals into groups or maintaining
a closed herd can be an effective strategy. Quarantine of any new arrivals onto the farm for a minimum of 30 days is also highly recommended4 as is investigation into the horses vaccination status, health certificate and negative tests results. All equipment used for new arrivals should be separate or disinfected and cleaned thoroughly if shared around the property.4 Any personal involved in handling said animals, should also clean their bodies, boots and clothing thoroughly before handling other animals. Vaccines currently used include, inert or live vaccines. Current guidelines are to vaccinate foals over 3-5 months of age, with a booster within 4-6 weeks, along with vaccination every 3-6 months to enhance immunity.4 Pregnant mares should also be vaccinated at the fifth, seventh, and ninth months of pregnancy.4
Contributing Institution:
Massey University
School of Veterinary Science
Private Bag 11 222
Palmerston North 4442
New Zealand
JPC Diagnosis:
Liver: Hepatitis, necrotizing, subacute, multifocal, random, moderate, with hetpacellular intranuclear viral inclusions.
JPC Comment:
This week’s session was led by Colonel Sherri Daye, Director of JPC Veterinary Pathology (and all-around equine enthusiast). The cases for the first conference of WSC 2024-2025 were entirely an ‘equine affaire’ with 4 classic entities that have been seen in the conference before. This first particular case is diagnostically rewarding with multifocal, random areas of necrosis within the liver that provide a solid hint at the underlying pathogenesis. Likewise, intranuclear viral inclusions within hepatocytes are characteristic (figure 1-3) and help refine differentials for this case. Conference participants remarked at the distribution of necrosis in this case – there was a brief discussion of whether coagulative necrosis secondary to viral ischemic effects was a significant feature of this case. Ultimately, the group felt that endothelial cells lacked obvious viral inclusions and that the lesions were not predominately vasocentric and lacked significant thrombi. Additionally, the group slightly differed from the contributor in chronicity, noting that most cases of abortion take an extended period of time to develop given the cell-to-cell spread of alphaherpesviruses.1 Moreover, a similar case of EHV in a neonatal horse was reviewed in a previous WSC (Conference 7, Case 3, 2015-2016) with extensive regions of hemorrhage and lytic necrosis within the lung, liver, and adrenal gland that reflects the expected distribution of lesions in animals that may be infected late in term and/or survive to parturition with EHV. Absent confirmation of the diagnosis via PCR and/or IHC of tissue in this case, we are left to favor EHV-1 over EHV-4.
The case contributor provides a nice overview of equine herpesviruses with emphasis on their role in equine abortion. Prevention of EHV-associated disease remains paramount; current consensus recommendations were recently updated by the ACVIM6 and include vaccination in conjunction with a robust biosecurity program. To date, vaccination alone is not sufficient to prevent spread of EHV nor does it ameliorate clinical signs in sick horses. That stated, the ability of vaccines to prevent the development of viremia, at least in some animals, can serve to avoid devastating sequalae such as abortion or myeloencephalopathy.6
There are a number of other potential causes for abortion and stillbirth in foals. Bacterial causes include beta-hemolytic streptococci, E. coli, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, and Actinobacillus equuli among others.1, 7 Infection may occur as a consequence of mare bacteremia or, more commonly, via ascending infection through the cervix. Fungal causes are typically attributed to Aspergillus spp., though other organisms may occasionally be isolated.1,7 Rarely, equine viral arteritis has been isolated in abortion cases.1,7 Non-infectious causes such as umbilical cord torsion and congenital malformation also remain common. Definitive cause of abortion and stillbirths remains elusive in many cases with only 29.2% of all submissions to a California diagnostic lab having a certain etiology identified.1
Finally, EHV-1 and EHV-4 should be distinguished from EHV-2 and EHV-5 which are both gamma herpesviruses that do not cause abortion, but are isolated both from healthy horses and those with equine multinodular pulmonary fibrosis (EMPF; EHV-5) and respiratory illness (EHV-2). There are multiple examples of EHV-5 in previous WSC proceedings. EHV-3 is associated with equine coital exanthema, and rarely, subclinical respiratory infections in yearling horses.7
References:
- Cantón GJ, Navarro MA, Asin J, et al. Equine abortion and stillbirth in California: a review of 1,774 cases received at a diagnostic laboratory, 1990-2022. J Vet Diagn Invest. 2023 Mar;35(2):153-162.
- Dunowska M. A review of equid herpesvirus 1 for the veterinary practitioner. Part A: clinical presentation, diagnosis, and treatment. New Zeal Vet J. 2014;62: 171-178.
- Dunowska M. A review of equid herpesvirus 1 for the veterinary practitioner. Part B: pathogenesis and epidemiology. New Zeal Vet J. 2014;62: 179-188.
- Khusro A, Aarti C, Rivas-Caceres RR, Barbabosa-Pliego A. Equine herpesvirus-1 infection in horses: Recent updates on its pathogenicity, vaccination, and preventive management strategies. J Equine Vet Sci. 2020;87: 102923.
- Laval K, Poelaert KCK, Van Cleemput J, et al. The pathogenesis and immune evasive mechanisms of equine herpesvirus type 1. Front Microbiol. 2021;12: 662686.
- Lunn DP, Burgess BA, Dorman DC, et al. Updated ACVIM consensus statement on equine herpesvirus-1. J Vet Intern Med. 2024 May-Jun;38(3):1290-1299.
- Schlafer DH, Foster RA. Chapter 4: Female genital system. ln: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Missouri, USA: Elsevier; 2016:358-464.
- Smith KC. Herpesviral abortion in domestic animals. Vet J. 1997;153: 253-268.
- Smith KC, Blunden AS, Whitewell KE, Dunn KA, Wales AD. A survey of equine abortion, stillbirth and neonatal death in the UK from 1988 to 1997. Equine Vet. J. 2003;35: 496-501.
- Van Maanen C. Equine herpes virus 1 and 4 infections: an update. Vet Q. 2002;24: 57-78.