Signalment:
2-year-old male beagle dogThis animal was part of an IACUC approved animal study to evaluate the pathogenesis and
treatment of septic shock. The dog had been anesthetized and an intrabronchial inoculation of
Staphylococcus aureus was placed into the right caudal lobe. The dog was sedated with fentanyl, versed
and medetomidine and was mechanically ventilated via an endotracheally tube for 89 hours. Intravenous
fluid administration with Normosol was provided to maintain blood pressure and hydration. Oxygen was
administered to maintain adequate arterial oxygenation. After 24 hours reduced arterial oxygenation
required the level of oxygen administration to be increased to 100%. The dog died after 89 hours, seven
hours before the termination point for the study. The post-mortem interval was 10 hours at refrigeration
temperature.
Gross Description:
At necropsy this animal was well muscled with a moderate amount of body fat and in
good hydration. Edema was noted in the subcutaneous tissues of the ventral neck and thorax.
Approximately 500 ml of clear serosanguineous fluid was present in the abdominal cavity and
approximately 400 ml of similar fluid was present in the thoracic cavity. The lungs were moderately
atelectatic secondary to the presence of the pleural effusion. An area within the right caudal lobe measuring
approximately 3 cm x 3 cm was noted to be pale with demarcated borders consistent with a focus of
necrosis, which corresponded to the placement of the bacterial clot. All lung lobes were firm and sank in
formalin. Multifocal petechial and ecchymotic hemorrhages were noted in the pancreas and mesentery.
The liver, kidneys and spleen were moderately congested. The remaining organs and tissues appeared
normal.
Histopathologic Description:
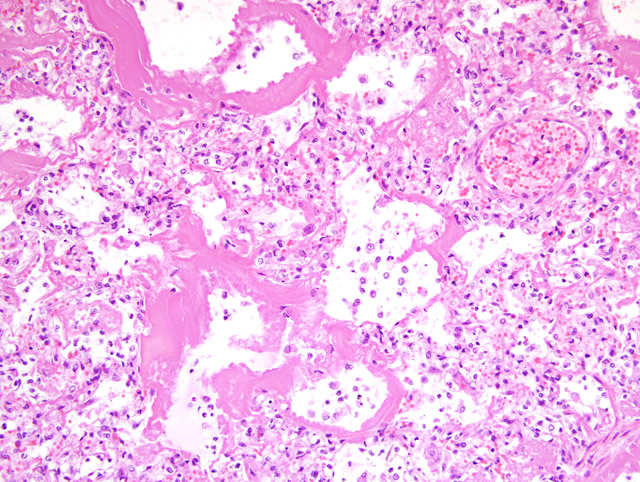
There was a severe fibrinosuppurative bronchopneumonia. Bronchioles
and alveoli contained large numbers of neutrophils admixed with moderate numbers of alveolar
macrophages and fibrin. The bronchi, bronchioles and alveoli were prominently lined by homogeneous
eosinophilic granular to fibrillar hyaline membranes (
Fig. 1-1). Degenerative cells could be identified
within the hyaline membranes in areas. Respiratory epithelium was not evident lining the bronchioles.
Morphologic Diagnosis:
1. Lung: bronchopneumonia, fibrinosuppurative, severe
2. Lung: hyaline membranes, bronchiolar and alveolar, diffuse
3. Multiple organs: bacteremia, bacterial rods
Lab Results:
Samples for bacterial culture were collected from the femoral and jugular catheters,
which yielded a mixed growth of Klebsiella pneumonia and Acinetobacter baumannii.
Condition:
Acute respiratory distress syndrome
Contributor Comment:
The suppurative bronchopneumonia associated with intrabronchial
administration of Staphylococcus aureus in this case was similar to other dogs examined on this protocol.
The atypical feature of the histologic appearance of the bronchopneumonia in this case was the presence of
prominent bronchiolar and alveolar hyaline membranes. Hyaline membranes may occur in a variety of
disease entities where there is diffuse alveolar damage. In premature infants there is a condition termed
hyaline membrane disease of the newborn or respiratory distress syndrome. Hyaline membranes are also a
common feature of the Acute Respiratory Distress Syndrome (ARDS). Hyaline membranes are comprised
of homogenous granular or fibrillar eosinophilic material, which line alveoli and bronchioles. They are
composed of necrotic epithelial cell debris admixed with fibrin and plasma elements.(9)
Immunohistochemistry studies in human tissues have demonstrated that the epithelial and endothelial
components of surfactant apoprotein A, factor VIII related antigen and cytokeratin AE1/AE3 are present in
hyaline membranes associated with diffuse alveolar damage.(8)
Respiratory distress syndrome in premature infants is associated with inadequate levels of pulmonary
surfactant produced by type II pneumocytes.(5) Decreased levels of surfactant causes increased alveolar
surface tension, which leads to atelectasis, hypoxemia and acidosis. This further causes pulmonary
vasoconstriction and hypoperfusion leading to capillary endothelial damage, plasma leakage, fibrin
deposition and hyaline membrane formation.
Acute respiratory distress syndrome occurs in a variety of mammalian species including man. ARDS can
be due to a variety of etiologic factors such as septic shock, physiologic shock associated with trauma or
burns, severe pulmonary viral infections such as SARS, inhaled toxins or irritants such as smoke, phosgene
and mercury vapor, hypersensitivity to certain organic solvents and herbicides such as kerosene and
paraquat, high altitude, cytotoxic drugs such as bleomycin, busulfan and methotrexate, and oxygen toxicity.
(1) The common pathogenesis in these entities is the development of acute diffuse alveolar damage to the
alveolar epithelium and capillary endothelium with interstitial and intraalveolar edema and fibrin
exudation and the development of hyaline membranes.(6) As a response to the alveolar injury, type II
epithelial cells will proliferate and resolution will either lead to recovery or given the severity of the injury
may lead to pulmonary fibrosis.
In this case there were multiple interrelated contributing factors, which may have led to the development of
pulmonary hyaline membranes, including septic shock, terminal gram negative sepsis, mechanical
ventilation injury and oxygen toxicity. The most likely significant cause was oxygen toxicity. Oxygen
toxicity has been induced and has been reported to occur in a wide variety of mammalian species.
Exposure to oxygen levels of 85-100% for a prolonged period can cause oxygen toxicity. Dogs exposed to
1 atm of oxygen had an average survival time of approximately 60-80 hours.(3) Oxygen derived free
radicals including superoxide, hydroxyl ion and singlet oxygen can directly injure cell membrane by
causing lipid peroxidation. Additionally, there is inhibition of nucleic acid and protein synthesis and
inactivation of cellular enzymes. Damage to pulmonary epithelium may also lead to decreased levels of
surfactant. Oxygen toxicity can also induce CNS signs of vertigo and convulsions.(7) Ultrastructurally,
studies have shown that as little as 1 to 4 hours of exposure to 100% oxygen can cause morphologic
changes to type I epithelial cells with bleb formation of the cytoplasmic membranes and swelling of
endothelial cells with plasma transudation.(4)
JPC Diagnosis:
Lung: Pneumonia, bronchointerstitial, fibrinosuppurative, acute, diffuse, severe with
bronchiolar and alveolar hyaline membranes and bacteria.
Conference Comment:
The contributor gave an excellent explanation of both the cause and pathogenesis
of ARDS. Grossly, lungs with this type of insult contain lesions with greater involvement of the
dorsocaudal lung fields. Despite the nature of the causative agent, diffuse alveolar damage leads to a
predictable histologic pattern of progression from an acute exudative phase to a subacute proliferative
phase followed by a chronic fibrosing phase.(2)
References:
1. Blennerhassett JB. Shock lung and diffuse alveolar damage pathological and pathogenetic
considerations. Pathology
17(2):239-47, 1985
2. Caswell JL, Williams KJ: Respiratory system.Â
In: Jubb, Kennedy and Palmers Pathology of Domestic
Animals, ed. Maxie MG, 5th ed., vol 2, pp.564-567. Elsevier Limited, Edinburgh, UK, 2007
3. Clark JM, Lambertsen CJ: Pulmonary oxygen toxicity: a review. Pharmacol Rev
23(2):37-133, 1971
4. Coalson JJ, Beller JJ, Greenfield LJ: Effects of 100 per cent oxygen ventilation on pulmonary
ultrastructure and mechanics. J Pathol
104(4):267-73, 1971
5. Hallman M, Glumoff V, Ramet M: Surfactant in respiratory distress syndrome and lung injury. Comp
Biochem Physiol A Mol Integr Physiol
129(1):287-94, 2001
6. Hasleton PS, Roberts TE: Adult respiratory distress syndrome- an update. Histopathology
34(4):285-94,
1999
7. Patel DN, Goel A,, Agarwal SB, Garg P, Lakhani KK: Oxygen toxicity. J Indian Academy of Internal
Medicine
4(3):234-7, 2003
8. Peres SA, Parra ER, Eher E, Capelozzi VL: Nonhomogenous immunostaining of hyaline membranes in
different manifestations of diffuse alveolar damage. Clinics
61(6):497-502, 2006
9. Scarpelli EM: Respiratory distress syndrome of the newborn. Annu Rev Med
19:153-166,1968