Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 19
30 January, 2019
CASE I: TAMU-2 2014 (JPC 4054746).
Signalment: Adult, female, Leopard Tortoise (Stigmochelys pardalis)
History: Animal presented to clinic for dyspnea, oral/ocular/nasal discharge, lethargy, anorexia and mucoid cloacal discharge. Eyes matted shut. No fecal/urinary production. This recently purchased tortoise arrived emaciated and anorectic with oculonasal discharge. Animal had anemia, hyperuricemia, hypercalcemia and hyperkalemia, and renal disease was suspected. Radiographs indicated possible pneumonia. Treated for 3 days, when owner opted for humane euthanasia.
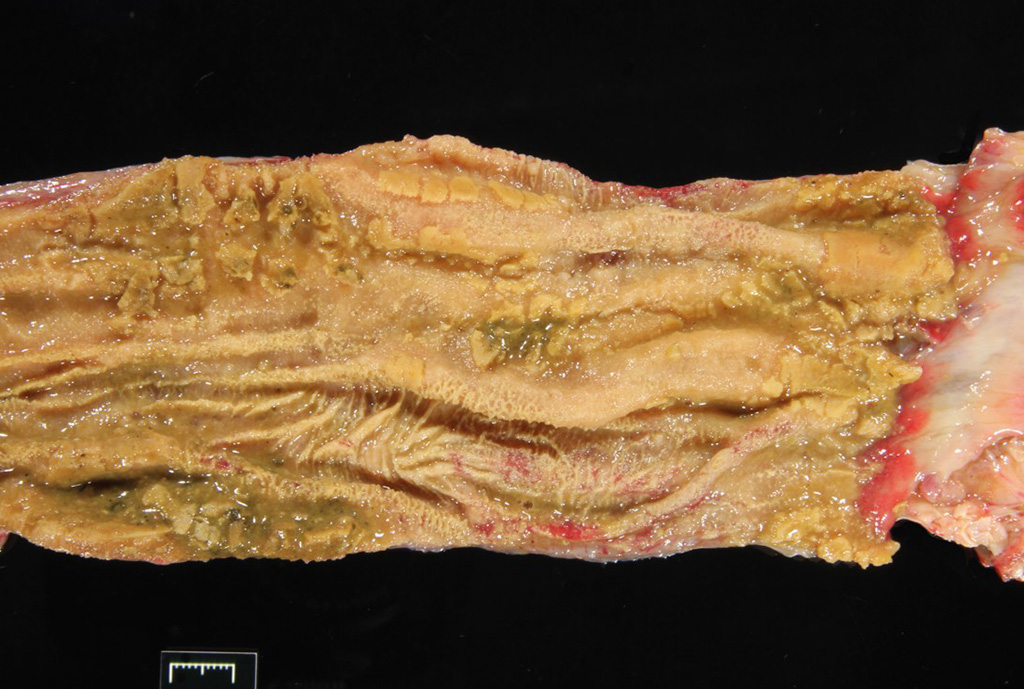
Gross Pathology: The carapace had “pyramiding” indicating metabolic/nutritional disease. Other significant gross lesions included: fat pad loss (emaciation), severe enterocolitis, hepatic lipidosis, pleuritis and pulmonary emphysema, and bilateral conjunctivitis.
Laboratory results: PCV 17 with ++ polychromasia
WBC- 9500, 69% heterophils, 13% lymphocytes, 15% monocytes, 2% eosinophils 1% basophils
K+ 8.5 mmol/L, Uric Acid >25mg/dL, Bile Acids= 35 umol/L, Ca+ 20 mg/dL
Microscopic Description:
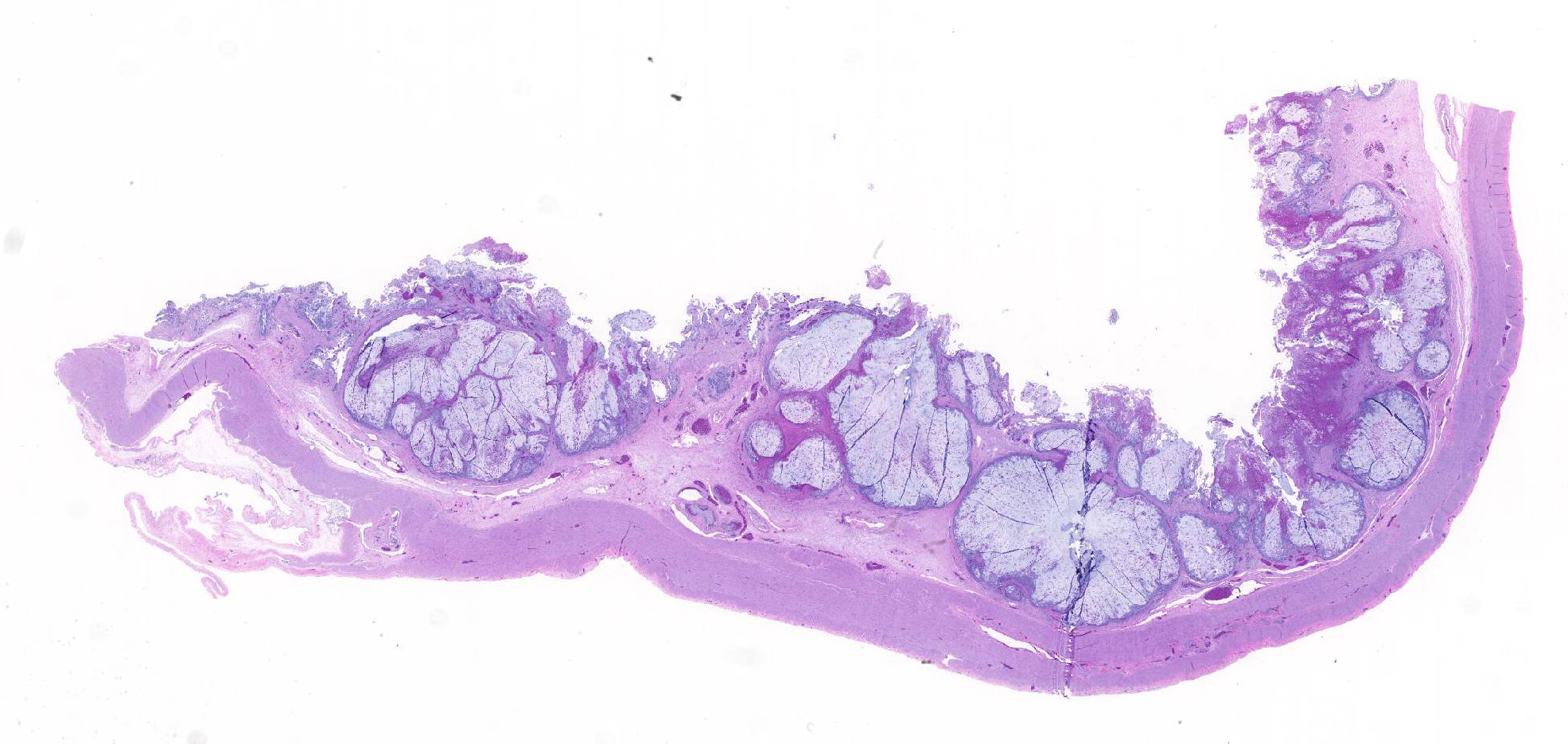
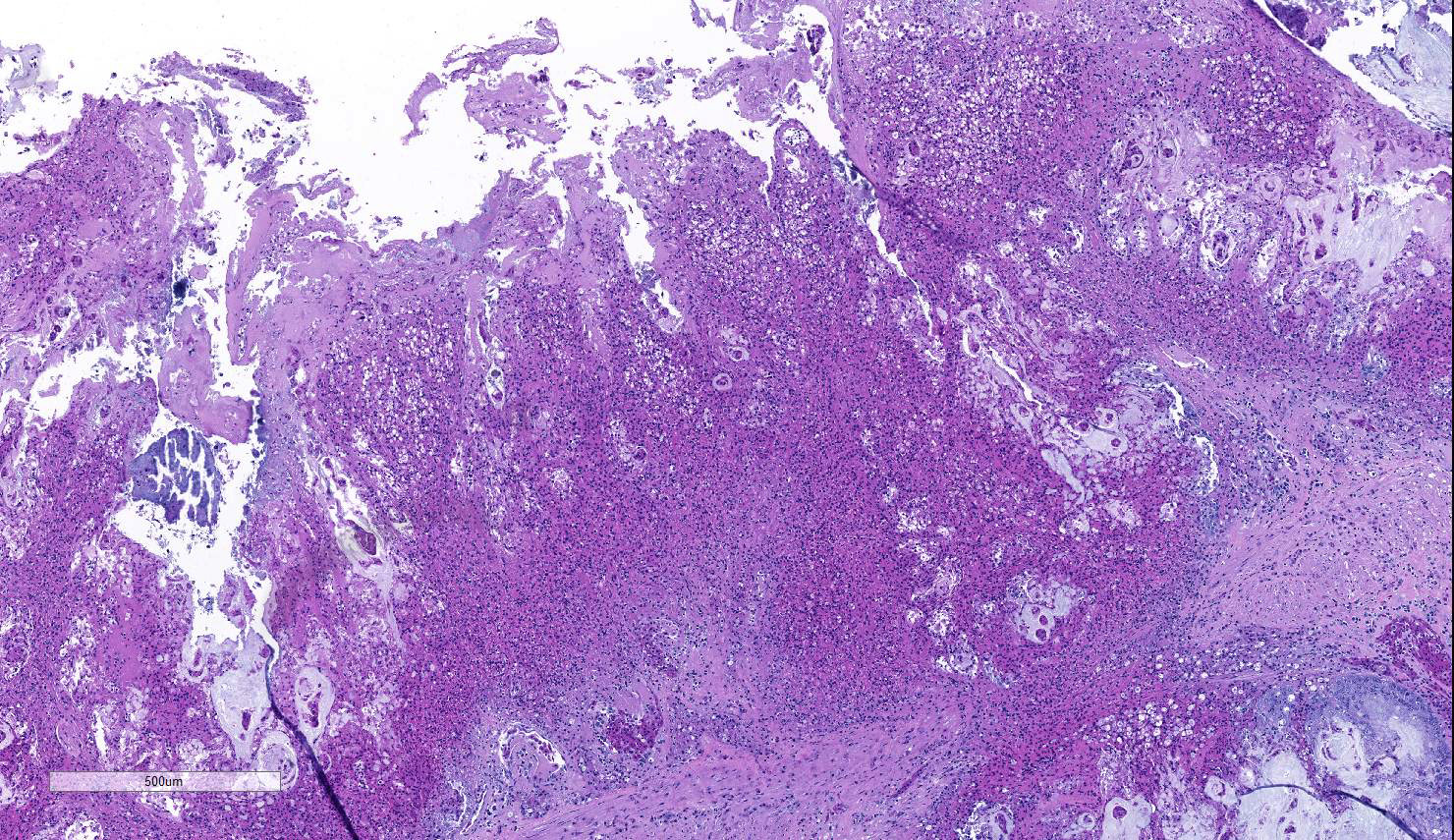
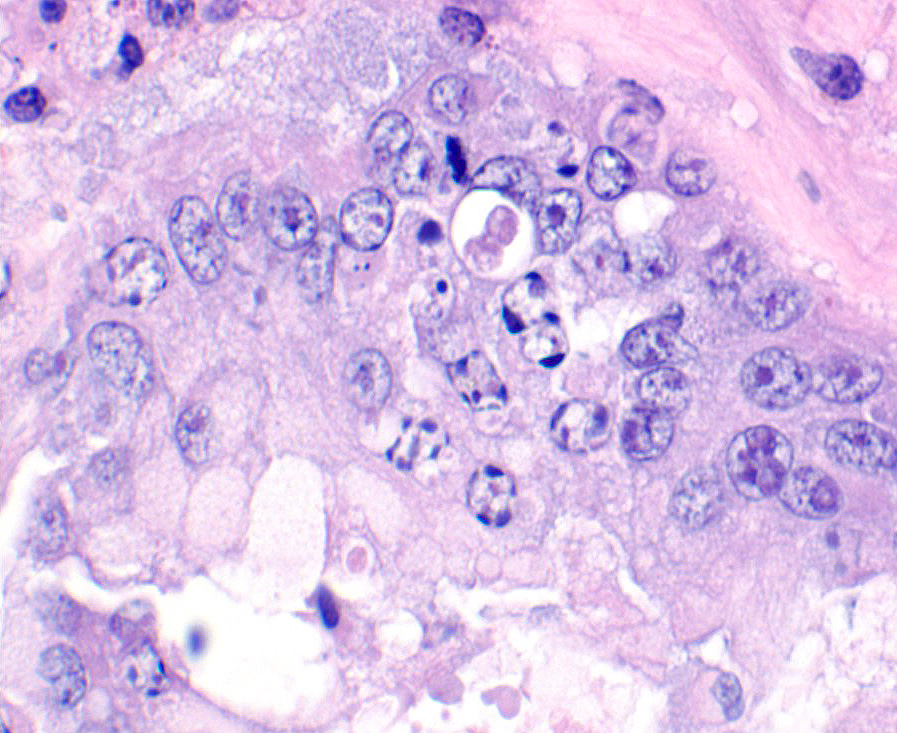
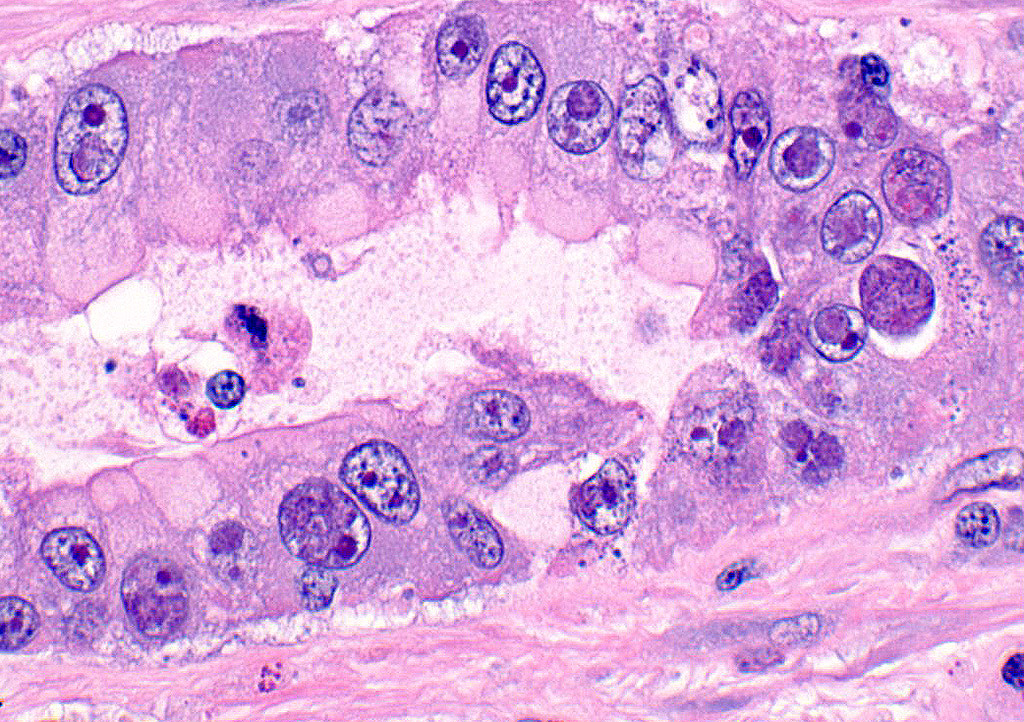
A section of terminal colon is presented. The long folds are an anatomic normal. Large segments of mucosa, especially the length of the folds, are covered by necrotic mucosa, cell debris and heterophils and macrophages, and saccules of the colon are distended by mucus with a mild, necrotic, pleocellular detritis and numerous bacteria. Viable mucosa is disorganized and not the normal, simple, columnar epithelium. Colonic epithelium is piling up. Cells have large, hypochromic nuclei with prominent nucleoli and with somewhat granular, poorly defined eosinophilic cytoplasm. Small numbers of granulocytes exocytose. The submucosa has distended vessels (congestion) with some extravasated erythrocytes, and tissues are rarified (edema) with a mild to moderate granulocytic, histiocytic and lymphocytic infiltrate. The muscularis is intact, and coelomitis is not noted.
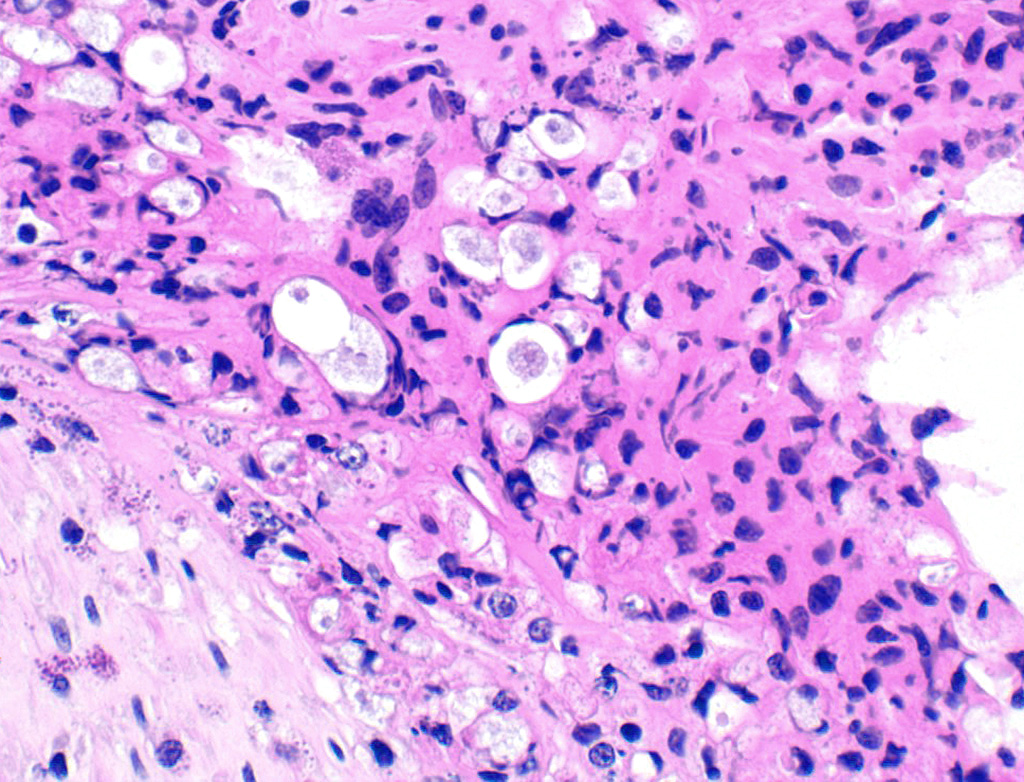
In the necrotic cell debris and in the viable epithelium are many, 20-35u amoeba trophozoites with usually a single endosome and granular or globular cytoplasm (Entamoeba sp.). Additionally, many of the epithelial nuclei have variable numbers (1 to 8) of eosinophilic meronts. Pairs and clusters of these meronts, and clusters like merozoites of these coccidian are noted. Rare megaschizonts may be seen.
Contributor’s Morphologic Diagnoses:
Necrogranulocytic/heterophilic, hyperplastic and mucinous colitis with amoeba and intranuclear coccidian.
Contributor’s Comment: This is apparently a common presentation in land tortoises, based on cases published. 3 The animal was euthanized; however, she had a severe enterocolitis. The severity of the GI lesion and the two associated pathogens were the reasons for the choice of our tissue selection. The coexistence of the presumed Entamoeba and intranuclear coccidian is not unique to our case, because it is reported.
Because no bacterial pathogens or their toxins (Salmonella, Clostridium, Citrobacter etc.,) were recovered from this tortoise, the Entamoeba was considered a significant pathogen. 2,6,9,13 Entamoeba invadens has been isolated from a leopard tortoise, but it should be remembered that this organism is considered a commensal to most species of chelodons. Epizootics associated with large numbers of amoebae in reptiles are reported6, but many are not tested with molecular procedures. It has been reported that the morphology of amoeba especially the morphology of the trophozoite in tissue is not adequate to speciate this protozoa. 2,13 Diagnosticians must start testing, culturing and collecting fecal cysts and typing these organisms to establish bona fide pathogenic strains. It is interesting that seven species of tetranucleated-cyst-producing Entamoeba (E. invadens has 2-4 nuclei in cysts) have been associated with chelonian hosts.
In this particular case, deep ulcers were not seen in the colon, and amoeba were not associated with hepatic lesions; however, Entamoeba was presumed to be a significant pathogen in the colon, even though it did not burrow deeply because such large numbers of organisms are visible in viable colon as well as in the necrotic debris. One report of entamoebiasis in a leopard tortoise was associated with a myositis (not seen in our case).10 A similar, verminous colonic lesion has been reported associated with Proatractis sp., but no embedded parasites were seen in our case.10
Intranuclear coccidia are reported in chelodons.1,3,5,7,8,12 The identity of the intranuclear coccidian is not known, but one report suggests it is a member of the Sarcocystidae while another suggests their case organism may be closely related to an Eimeria. 1,5,12 Multisystemic infections similar to our case also are reported in leopard tortoises. The proliferative lesion of the colon is presumed to be due to the coccidian, perhaps similar to the proliferative lesion seen in sheep and goats with eimeriosis. Coccidia affected several systems in this individual. The colonic intranuclear forms are varied.3,5,7 The renal coccidial infection was striking in our case and no doubt was responsible for the renal compromise. The conjunctivitis was associated with coccidian as well: however, we did not culture for Mycoplasma (especially M. agassizii), a conjunctivitis-associated pathogen in tortoises. 7 The liver was lipidotic, and no cytoplasmic coccidial forms were seen in hepatocytes as reported in one published case, but intranuclear coccidians were occasionally seen in biliary epithelium. It is interesting to speculate that some nervous signs may be seen with coccidiosis as seen in cattle!
We did not test for either Type I or II chelodon herpesvirus, perhaps an oversight, but we were overwhelmed by the enterocolitis. Any herpesviral inclusions would be difficult to distinguish from intranuclear coccidian forms without EM or immunotesting. A causal relationship between chelodon herpesviruses and any of the herpesvirus related conditions is sometimes tenuous. The adenoviral nuclear inclusions, however, would seem to be distinguishable however.12
Contributing Institution:
Department of Veterinary Pathobiology
College of Vet Med and Biomedical Sciences
Texas A&M University
College Station, TX USA 77843-4467
JPC Diagnosis: Colon: Colitis, necrotizing, multifocal to coalescing, subacute, severe with numerous amebic trophozoites,, and intranuclear apicomplexan zoites and schizonts.
JPC Comment: Entamoeba invadens is a common parasite of reptiles which occasionally affects turtles. Within collections, freshwater turtles are considered to be a source of infection for other reptile species; snakes may be especially hard hit. The genus Entamoeba infects many classes of vertebrates, but only a few species have been proven to cause disease. Entamoeba suis has been identified in swine with hemorrhagic colitis. Several species of Entamoeba infect man and non-human primates: Entamoeba histolytica the cause of “amebic dysentery” and rare cases of extraintestinal disease, as well as non-pathogenic species E. dispar and E. moshkovii.12 Intestinal colonization occurs with encysted parasites and infection can be spread by asymptomatic carrier by oral-fecal routes. Excystation of the organism results in production of trophozoites which invade the intestinal wall leading to ulcer formation and diarrhea.12 Trophozoites are capable of penetrating the intestinal wall and can lead to more severe complications including liver abscesses (the most common) and, in rare cases, can spread to the brain and/or lungs, which is often fatal.12 Recent Entamoeba cases in the Wednesday Slide Conference include a case of E. invadens in the intestine of a green anaconda (WSC 2013-2014, Conf. 21, Case 4; and E. histolytica in the liver of a guereza monkey – WSC 2010-2011, Conference 6, Case 1.)
Intranuclear coccidia have been sporadically reported since the 1990s and found in a wide range of chelonians to include leopard, Sulawesi, and giant tortoises, among others, as well as Eastern box turtles.10 They are considered systemic infections and widespread in chelonian collections. Clinical presentation of this disease is variable and includes mucoid nasal and/or ocular discharge (seen in this case), and it may exist as a coinfection with amebiasis (seen here), mycoplasmosis, or bacterial septicemia. Gross lesions may include generalized subcutaneous edema, coelomic effusion, necrotizing enteritis, systemic petechiation, and skin ulcers. Intranuclear coccidia may be present in a wide range of cells, including enterocytes and renal tubular epithelium as seen in this case, hepatocytes, biliary epithelium, pancreatic ductular epithelium and acinar cells, urinary bladder epithelium, nasal mucosa, and tracheal epithelium and pneumocytes.10 Recently, oro-fecal transmission has been established as the method of transmission between individuals.4
While most commonly identified in chelonians, intranuclear coccidia may also been seen in other species. Cyclospora caryolytica and Cyclospora talpae are present within the small intestine and liver, respectively, of moles, and other species of Cyclospora have been identified as causing watery diarrhea in Japanese Black calves. 14 Eimeria alabamensis and E. subspherica have also been identified in diarrheal disease of calves, also including Japanese black calves (E. subspherica.14 Eimeria hermani and E. stigmosa have been observed in the enterocytes of geese.14
The moderator mentioned that the cause of pyramiding is actually due to low humidity, rather than nutritional causes (although a concurrent nutritional cause cannot be totally ruled out.)
References:
1. Alvarez WA, Gibbons PM, Rivera S, Archer LL, Childress AL, Wellehan Jr JFX, Development of a quantitative PCR for rapid and sensitive diagnosis of an intranuclear coccidian parasite in Testudines (TINC), and detection in the critically endangered Arakan forest turtle (Heosemys depressa). Vet Parasitol 193: 66-70, 2013
2. Bradford CM, Denver MC, Cranfield MR: Development of a polymerase chain reaction test for Entamoeba invadens. J Zoo Wildl Med 39: 201-7, 2008
3. Garner MM, Gardiner CH, Wellehan JFX, Johnson AJ, McNamara T, Linn M, Terrell SP, Childress A, Jacobson ER
: Intranuclear coccidiosis in tortoises: Nine cases. Vet Pathol 43: 311-20 (2006)
4. Hoffmannova L, Kvicerova J, Bizkova K, Modry D. Intranuclear coccidiosis in tortoises – discovery of its causative agent and transmission. Eur J Protistol 2019; 67:71-76.
5. Innis CJ, Garner MM, Johnson AJ, Wellehan JFX, Tabaka C, Marschang RE, Nordhausen RW, Jacobson ER: Antemortem diagnosis and characterization of nasal intranuclear coccidiosis in Sulawesi tortoise (Indotestudo forsteni). J Vet Diagn Invest 19: 660-7, 2007
6. Jacobson ER, Schumacher J, Telford SR Jr, Greiner EC, Buergelt CD, Gardiner CH: Intranuclear coccidiosis in radiated tortoises (Geochelone radiata). J Zoo Wildl Med 25:95–102, 1994
7. Jacobson ER, Causes of mortality and disease in tortoises: a review. J Zoo and Wildlife Medicine 25: 2-17, 1994
8. Origgi FC, Jacobson ER: Diseases of the respiratory tract of chelonians. Vet Clin No Amer Exotic Anim Prac 3:537-49, 2000
9. Philbey AW: Amoebic enterocolitis and acute myonecrosis in leopard tortoises (Geochelone pardalis). Vet Rec 158:567-69, 2006
9. Rideout BA, Montali RJ, Phillips LG, Gardiner CH: Mortality of captive tortoises due to viviparous nematodes of the genus Proatractis (Family Atractidae). J Wildl Dis 23: 103-8, 1987
10. Rodriguez CE, Duque AMH, Steinberg J, Woodburn DB. Chelonia. In Terio KA, McAloose St. Leger J, eds. Pathology of Wildlife and Zoo Animals. London: Elsevier Inc. pp 819-847.
11. Schmidt V, Dyachenko V, Aupperle H, Pees M, Krautwald-Junghanns EM, Daugschies A: Case report of systemic coccidiosis in a radiated tortoise (Geochelone radiata). Parasitol Res 102: 431-36, 2008
12. Skappak C, Akierman S, Belga S, Novak K, Chadee K, Urbanski SJ, Church D, Beck PL. Invasive amoebiasis: A review of Entameoba infection highlighted with case reports. Can J Gastroenterol Hepatol 2014; 28(7):355-359.
13. Stensvold CR, Lebbad M, Victory EL, Verweij JJ,
Tannich E, Alfellani M, Legarraga P, and Clark CG: Increased sampling reveals novel lineages of Entamoeba: Consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist 162: 525-41, 2001
14. Yamada M, Hatama S, Ishikawa Y, Kadota K. Intranuclear coccidiois caused by Cyclospora spp. in calves. J Vet Diagn Invest 2014; 26(5):678-682.