Signalment:
Gross Description:
Note: The larvae collected from the pasture where deaths occurred were ground and orally fed to an unrelated calf of the same age (18-month-old) in one single dose of 20 g/kg/body weight. The calf develop clinical signs similar to the ones described above within 3 days after the administration of the larvae and died after a short period (approximately 24 hours) of the onset of clinical disease. The serum activity of liver enzymes was increased as measured before administration of the larvae and just prior the death of the experimental calf. Results of the two evaluated samples are within parenthesis (first value is from the pre-experimental sample and the second value is from the blood sampled just prior the death of the calf). Aspartate aminotransferase (73-804 U/L); gamma-glutamyl transferase (10-89 U/L); and alkaline phosphatase (54-404 U/L). The levels of total bilirubin were also elevated (3.46 mg/dL) and direct bilirubin elevation (2.98 mg/dL) accounted for this increase. Necropsy and histopathological findings on this experimental calf were similar to the ones observed in the spontaneous outbreak but were included in this submission.
Histopathologic Description:
Morphologic Diagnosis:
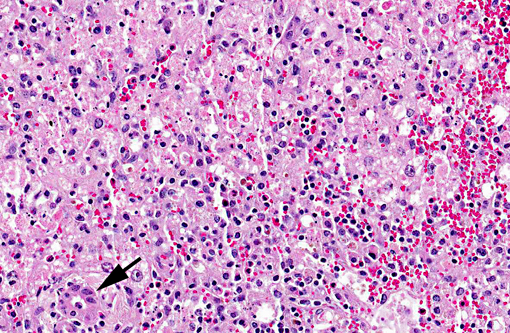
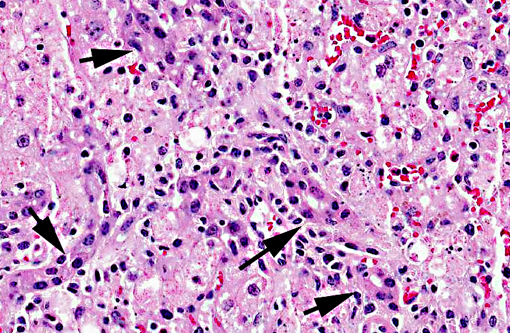
Liver, centrilobular to massive hepatocellular necrosis, diffuse, acute, and severe.
Etiologic diagnosis: Toxic hepatopathy
Etiology: ingestion of sawfly (Perreyia flavipes,)
Condition:
Contributor Comment:
In Australia, SLP is caused by the ingestion of the larvae of the sawfly Lophyrotoma interrupta (Pergidae, Hymenoptera), formerly referred also as Pterigophorus analis, interrupts interrupta and Platysectra interrupta.(8,17) The poisoning of cattle by eating L. interrupta (commonly referred to as Australian sawfly larva) has been studied for over 70 years, since it was first described in the Maranoa district of southern Queensland in 1911(2,8,17,22) where it reportedly is responsible for an annual loss of 1 million Australian dollars (1981 values).(8) During 1972-1981 in Australia, 37 farms experienced 5,254 deaths during July to September and 1,800 deaths in cattle occurring in just one year were attributed to the intoxication.(4) Cattle were introduced in this area between 1862 and 1866 and the first suspected cases occurred in 1887 but the definite occurrence was only established in 1911.(22) Outbreaks in Australia are confined to districts where there are large forests of the silver leaf ironbark tree (Eucalyptus melanoplhoia), the main host for L. interrupta(2,8,11,12) and most deaths occurred between July and October and some deaths may occur after the dried-out remains of dead larvae are moistened by rain during summer.(4)
In Denmark SLP was reported in sheep caused by the ingestion of the larvae of sawfly Arge pullata (Argidae), commonly referred as Danish sawfly larva which feed on birch trees (Betula pendula). This outbreak of SLP have been reported from the Danish Island of Sjelland where 50 sheep from a flock of 250 died 3 days after they were moved to the area infested by A. pullata larvae.(21) At this occasion, the disease was reproduced experimentally in goats that were fed larvae from this outbreak.(21)
The sawfly Perreyia flavipes Konow, 1899 (Pergidae: Perreyiinae) also referred as Lophyroides flavipes and Brachytoma flavipes have been reported from Argentina, Uruguay and Brazil in cattle,(5,15,19) sheep(5,14) and pigs(6) and the disease was reproduced in cattle, sheep(5) and pigs(18). P. flavipes is commonly referred to as South American sawfly larva.Â
The economical importance of sawfly (P. flavipes) larval poisoning in South America can be evaluated if one considers that within a three-year period (1993-1995) at least 40 outbreaks of this intoxication occurred in Uruguay and that during just one year (1995) cattle losses exceeded 1,000 heads.(5) Mortality rates vary and are reported as 1.6%, 7.0% and 1.38% from one study(5) and 0.8%, 6.2% and 33% from another.(19)
D-amino acid-containing peptides have been found to the toxic principle in each sawfly involved in farm animal poisonings.(7,9,10) The octapeptide lophyrotomin is the major toxin in the in the larvae of Australian and Danish sawflies and is present in small amounts in the larvae of South American sawfly. The heptadecapeptide pergidin is the main toxin in the South American sawfly while small amounts of pergidin have been found in the other two species of toxic sawfly.(9)
One interesting environmental phenomenon related to sawfly occurred in Florida. The broad-leaf paper bark tree Melaneuca quinquenervia was introduced from Australia into Florida, USA, early in the1900s, and has since then proliferated to such an extent as to be found in all 10 counties of Southern Florida in an area over 200,000 hectares where it causes considerable environmental and economic damage. Therefore the sawfly Lophyrotoma zonalis was introduced, again from Australia, as a possible biological control agent for this tree due to its ability to defoliate M. quinquenervia. However this may turn out to be a dangerous practice since, although no cases of spontaneous poisonings in animals attributable to the ingestion of L. zonalis larvae have been detected either from Florida or Australia, the toxins lophyrotomin and a mixture of pergidin and val4- pergidin were demonstrated in L. zonalis larvae(12), making the larvae a possible threat to livestock. Furthermore, the toxic substances in L. zonalis larvae proved toxic to mice.(10)
The biological cycle of P. flavipes was studied in the laboratory(18) and it was determined that it develops along the whole year. Larvae appear in pasture form March on, when they are bright black and small 1 mm in length thus being not promptly observed. From March to August (autumn and winter in the south hemisphere) they measure 17-22 mm in length and are promptly detected. Under normal conditions full growth is reached in the late winter and early spring. They are ingested by cattle during this period. The reason that cattle eat the sawfly larvae is unknown. It has been suggested that this behavior reflects some form of nutritional deficiency (Roberts 1932) however this remains unproven. Alternatively it has been suggested that some property of the sawfly may be involved in causing the animals to seek out and eat the larvae.(10) The nature of such attraction has not been determined, but it may also explain the existence of toxic areas.(10) Larvae of P. flavipes feed on decomposing plant material, dry leaves and dried cattle manure. Larvae go through a series of changes until they pupate, when the insect penetrates 3-10cm into the ground and form a bright black, leathery-like, 1x0.5 cm ovoid cocoon. Within the cocoon the larvae becomes white and remains in this stage from August to January when they emerge as adults. The adult insects are bright black and have a short life span: Only18-36 hours for females and 24-48 hours for males, time sufficient to start a new cycle. Females are 8-10 mm and males are 7.5-10 mm in length.(18)
The SLP is an acute condition in all affected species. Most affected cattle have weakness, muscular tremors, apathy, stupor and death within 2-5 days of the onset of clinical signs. (4,5,8,15) Some animals become very agitated and aggressive(5) and those cattle surviving for longer periods may show hepatogenous photo dermatitis.(2,5) Some less affected cattle may recover.(2,5,17)
Necropsy findings include accentuation of the lobular hepatic pattern, edema of the gall bladder and serosal hemorrhages. Fragments of larvae (P. flavipes) can be found in the rumen and omasum. The main histological lesion consists of centrilobular to massive liver necrosis and necrosis of lymphoid tissue.(5,15) Reportedly in cattle, necrosis of hepatocytes was most severe in the right lobe.(5) In our experience this is not an unusual finding in acute hepatotoxicosis in cattle and we think it may be related to differences in the input of blood supply to different regions of the liver.Â
The acute centrilobular necrosis as seen associated to SLP is not specific of this condition and occurs in association with several other hepatotoxins, mainly phytotoxins, in farm animals.(16) Hepatocytes in the center of the lobule (zone 3) are more vulnerable to a toxic insult as compared with peripheral (zone 1) located hepatocytes because centrilobular hepatocytes have more abundant enzymes which act to transform liposoluble compounds in toxic substances and because centrilobular hepatocytes have lower levels of oxygen and glutathione peroxidase. Hepatocytes in the periphery of the lobule (zone 1) are more vulnerable to toxins of direct action, due to the proximity of these periportal hepatocytes to the blood flow that arrives but the portal vein and hepatic artery branches at the portal triads.(20) Interestingly when the toxic principles of sawfly were administered to mice treated with lophyrotomin, they developed periportal (zone 1) necrosis and mice treated with pergidin developed centrilobular (zone 3) necrosis.(12) Regarding this aspect it is interesting to notice that a reduction in the sawfly-induced liver pathology associated as a consequence of the concurrent Fasciola hepatica infection was experimentally observed in lambs. Possible explanations for this phenomenon as an inhibitory action of F. hepatica on the microsomal oxidative enzymes responsible by the biotransformation of the active principles within the larvae in toxic metabolites.(13) Other hepatotoxins that affect livestock in Brazil are in Table 1.
The diagnosis in our case was based primarily in the clinical signs and pathological findings and on some epidemiological aspects. The aspects of the outbreak reported here are remarkably similar to SLP reported in cattle from Australia caused by L. interrupta, (2,8,17), in sheep from Denmark caused by Arge Pullata,(21) and in cattle and sheep from Uruguay(5) and cattle,(15,19), sheep(14) and pigs(6) from Brazil caused by P. flavipes. Further evidences include the finding of fragments of the larvae in the fore- stomachs of the necropsied calf and the reproduction of the disease by feeding ground P. flavipes larvae collected from the site where the spontaneous outbreak occurred to a susceptible calf.
The neurologic clinical signs presented by cattle of this report affected by SLP are typical of hepatic encephalopathy. Cattle poisoned by the larvae L. interrupta(8) and sheep fed Arge pulllata(21) also show signs of hepatic encephalopathy, and the fatally poisoned calves presented increased plasma ammonia sufficient to account for the clinical signs.(8) Hepatic encephalopathy is common in ruminants and horses with hepatic failure.(3) Undetermined as yet are the specific metabolites that cause the neurologic dysfunction in hepatic encephalopathy, but increased concentrations of plasma ammonia derived from amines absorbed from the gastrointestinal tract may be responsible.(3) Normally, amines are absorbed from the intestines into the portal blood and metabolized by the liver. The toxic products may not be fully eliminated by severely damaged liver. However, abnormal ammonia concentrations are not the only possible cause of hepatic encephalopathy. An imbalance between inhibitory and excitatory amino acid neurotransmitters, -aminobutyric acid, and L-glutamate, respectively, and increased brain concentrations of endogenous benzodiazepines are other possible explanations.(3) Alternatively a low blood sugar can account for the neurological signs and a fall in glucose was noticed in and the fatally poisoned calves by L. interrupta.(8)
Widespread hemorrhages are prominent in some field cases of SLP, but they were not invariably present and their severity varied. Lengthened thrombin, activated thromboplastin times and reduced fibrinogen concentration have been reported in calves experimentally poisoned with the larvae of Lophyrotoma interrupta.(8) Hemorrhagic diathesis occurs terminally in animals with severe liver necrosis.(3) In these cases bleeding tendencies associated with hepatic failure may be due to impaired synthesis of clotting factors, reduced clearance of the products of the clotting process, and metabolic abnormalities affecting platelet function that affect normal clotting, individually or in combination. In acute liver failure (as is the case of liver failure in SLP) diminished synthesis of clotting factors with a short half-life, such as factors V; VII, IX, and X, impairs the ability of blood to coagulate. Diminished clearance of fibrin degradation products activated coagulation factors, and plasminogen factors by the damaged liver also perturbs clotting. Metabolic disturbances resulting from liver failure can affect platelet function and lead to synthesis of abnormal fibrinogen, a condition termed dysfibrinogenemia.(3)
Due to the short course of the disease, jaundice and photosensitization were not common findings, although both were not were seen in some field cases. An increased concentration of serum bilirubin has been seen in experimental sawfly larval poisoning of cattle and sheep.(8,21)
Tubular and degeneration of the renal epithelium tubular as described in cattle,(2,19) sheep,(14) and pigs(18) were not observed in our cases.
Table1. Acute hepatotoxicosis in farm animals in Brazil
| Hepatoxin | Affected species | Toxic principle | Main lesion | Comments |
| Plants | ||||
| Xanthium spp. | Cattle, sheep, pigs | Carboxyatractylos+�-�de | Periacinar to massive necrosis | Poisoning in swine is associated with hypoglycemia and ascites |
| Cestrum parqui | Cattle | Carboxyatractylos+�-�de | Periacinar necrosis | |
| Cestrum corymbossum var. hirsute | Cattle | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. |
| Cestrum intermedium | Cattle | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. |
| Sessea brasiliensis | Cattle | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute. Experimentall small repeatedly administered doses can cause hepatic cirrhosis. |
| Dodonea viscose | Cattle | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. |
| Myoporum laetum | Sheep | Furanosesquiterpenoid oils (ngaione) | Usually periacinar necrosis but variable zonal necrosis can occcur. | Other species can be affected but the in Brazil was only recognized in sheep. |
| Cestrum intermedium | Cattle | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. |
| Cestrum laevigatum | Cattle | Saponins, cestrumide | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. Experimentall small repeatedly administered doses can cause hepatic cirrhosis. |
| Dodonea viscose | Cattle | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. |
| Trema micanthra | Goats and sheep | Not determined | Periacinar necrosis | Serosal hemorrhages, edema of the gall bladder wall. In natural conditions only causes acute poisoning. |
| Vernonia molissima | Cattle and sheep | Not determined | Periacinar necrosis | There is also degeneration of renal tubular epithelium |
| Vernonia rubricaulis | Cattle | Not determined | Necrose centrolobular | Outbreaks occur in the dry season |
| Bacteria | ||||
| Microcystis aeruginosa | Cattle, sheep, horses, goats | Microcystins and oithers | Periacinar to massive necrosis | Multiple toxins present. Can also cause death by neuromuscular disturbances. This poisoning was not doccumentsd in farm animals in Brazil but there are evidences that it occurs |
| Insect larvae | ||||
| Perreyia flavipeds (sawfly) | Cattle, sheep and pigs | Pergidin and lophyrotomin | Periacinar to massive necrosis | Serosal hemorrhages, edema of the gall bladder wall. |
| Mycotoxins | ||||
| Aflatoxin | Pigs, cattle | Bisfuranocoumarin compounds | Cnetrolobular necrosis (lipidosis) | Hemorrhages. Other species can be affected but the listed two are so more often in the country. Cattle are affecter as yound and develop a chronic form with fibrosis, megalocytosis and bile duct hyperplasia |
JPC Diagnosis:
Conference Comment:
Additionally, participants discussed the various toxins that can result in similar lesions of acute hepatic necrosis. The moderator provides the following table of agents involved in acute hepatic toxicity in cattle:
| Name | Toxic Principle |
| Blue-green algae | Microcystin-LR |
| Mushrooms: Amanita, Phalloides and others | Amatoxins |
| Cycads (Zamia sp.)Cycads (Zamia sp.) | Methylazoxymethanol |
| Solanacae (Cestrum sp.) | Atractyloside |
| Compositae (Xanthium-cockleburr) | Carboxyatractyloside |
| Ulmaceae (Trema sp.-Poison peach) | Trematoxin |
| Myoporacae (Myoporum) | Ngaione (periportal) |
| Iron | |
| Sawfly larvae (Lophyrotoma sp.) | Lophyrotomin/pergidin |
References:
2. Callow LL. Sawfly poisoning in cattle. Qld J Agric Sci. 1955; 81:155-161.
3. Cullen JM. Liver, biliary system, and exocrine pancreas, hepatic failure. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease, 5th ed. St. Louis Mosby:Elsevier, MO; 2007:408, 414.
4. Dadswell LP, Abbott WD, Mckenzie RA. The occurrence, cost and control of sawfly larval (Lophyrotoma interrupta) poisoning of cattle in Queensland 1972/81. Aust Vet J. 1984;62:94-97.
5. Dutra F, Riet-Correa F, Mendez MC, Paiva N. Poisoning of cattle and sheep in Uruguay by sawfly (Perreyia flavipes) larvae. Vet Hum Toxicol. 1997;39:281-286.
6. Jonck F, Casagrande RA, Froehlich DL, Ribeiro Jr DP, Gava A. Intoxica+�-�+�-�o espont+�-�nea por larvas de Perreyia flavipes (Pergidae) em su+�-�nos no Estado de Santa Catarina. [Spontaneous poisoning by larvae of Perreyia flavipes (Pergidae) in pigs in the State of Santa Catarina.] Pesq Vet Bras.2010;30:1017-1020. (In Portuguese, Abstract in English)
7. Kannan R, Oelrichs PB, Thamsborg ST, Willians DH. Identification of the octapeptide lophyrotomin in the European birch sawfly (Arge pullata). Toxicon. 1988;26:224-226.
8. McKenzie RA, Dunster PJ, Twist JO, Dimmock CK, Oelrichs PB, Rogers RJ, Reichmann KG. The toxicity of sawfly larvae (Lophyrotoma interrupta) to cattle. Qld. Dept. Prim. Ind. Bull. 1985; QB85001:1-48.
9. Oelrichs PB, MacLeod JK, Seawright AA, Moore MR, Ng JC, Dutra F, Riet-Correa F, M+�-�ndez MC & Thamsborg SM. Unique toxic peptides isolated from sawfly larvae in three continents. Toxicon. 1999;37:537-544.
10. Oelrichs PB, McLeod JK, Seawright AA, Grace PB. Isolation and identification of toxic peptides from Lophyrotoma zonalis (pergidae) sawfly larvae. Toxicon. 2001;39:1933-1936.
11. Oelrichs PB, Valley JV, McLeod JK, Cable J, Kiely D, Summers RE. Lophyrotomin, a new toxic octapeptide from the larvae of the sawfly, Lophyrotoma interrupta. Lloydia. 1977;40:209-214.
12. Oerlichs PB. Sawfly poisoning in cattle. Qld Agric J 108:110-112, 1982.
13. Olaechea FV, Thamsborg SM, Christensen N+�-�, Nansen P, Robles A. Interference with sawfly (Arge pullata) poisoning in Fasciola hepatica-infected lambs. J Comp Path. 1991;104:419-431
14. Raymundo DL, Bezerra Junior PS, Bandarra PM, Pedroso PMO, Oliveira EC, Pescador CA, Driemeier D. Spontaneous poisoning by larvae of Perreyia flavipes (Pergidae) in sheep. Pesq Vet Bras. 2008;28:169-173.
15. Raymundo D.L., Bezerra Jr P.S., Bandarra P.M., Santos A.S., Sonne L., Pavarini S.P., Correa A.M.R., Dias M.M. & Driemeier D. Intoxica+�-�+�-�o espont+�-�nea pelas larvas de Perreyia flavipes em bovinos no Estado de Santa Catarina, Brasil. Ci+�-�ncia Rural. 39:163-166, 2009.Â
16. Rissi DR, Driemeier D, Silva MC, Barros RR, Barros CSL. Poisonous plants producing acute hepatic disease in Brazilian cattle. In: Panter KE, Wierenga TL, Pfister JA, eds. Poisonous Plants: Global Research and Solutions. Wallingford, UK:CAB International; 2007:72-76.
17. Roberts FHS. The cattle-poisoning sawfly (Pterygophorus analis Costa). Qld Agric J. 1932;37:41-52,
18. Soares MP, Quevedo PS, Schild AL. Intoxica+�-�+�-�o por larvas de Perreyia flavipes em bovinos na Regi+�-�o Sul do Rio Grande do Sul. [Perreyia flavipes larvae poisoning in cattle in southern Rio Grande do Sul, Brazil.] Pesq Vet Bras. 2008;28:169-173. (In Portuguese, Abstract in English)
19. Soares MP, Riet-Correa F, Smith DR, Pereira Soares M, M+�-�ndez MC, Brandolt AL. Experimental intoxication by larvae of Perreyia flavipes Konow, 1899 (Hymenoptera: Pergidae) in pigs and some aspects on its biology. Toxicon. 2001;39:669-678.
20. Stalker MJ, Hayes MA. Liver and biliary system: toxic hepatic disease. In: Maxie MG. ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Saunders Elsevier. 2007:372-378.
21. Thamsborg SM, J+�-+rgensen RJ, Brummerstedt E. Sawfly poisoning in sheep and goats. Vet Rec. 1987;121:253-255.
22. Tryon H. Special cattle fatality in the Maranoa District, and its relation to the larvae of Pterygophorus analis, Costa. Qld Agric J. 1921;16. 208-216.