Signalment:
Gross Description:
A large gelatinous blood clot was present within the thoracic cavity on the right side. The lung lobes were soft, edematous, and congested. The right side was mildly to moderately affected. The left was mildly affected.
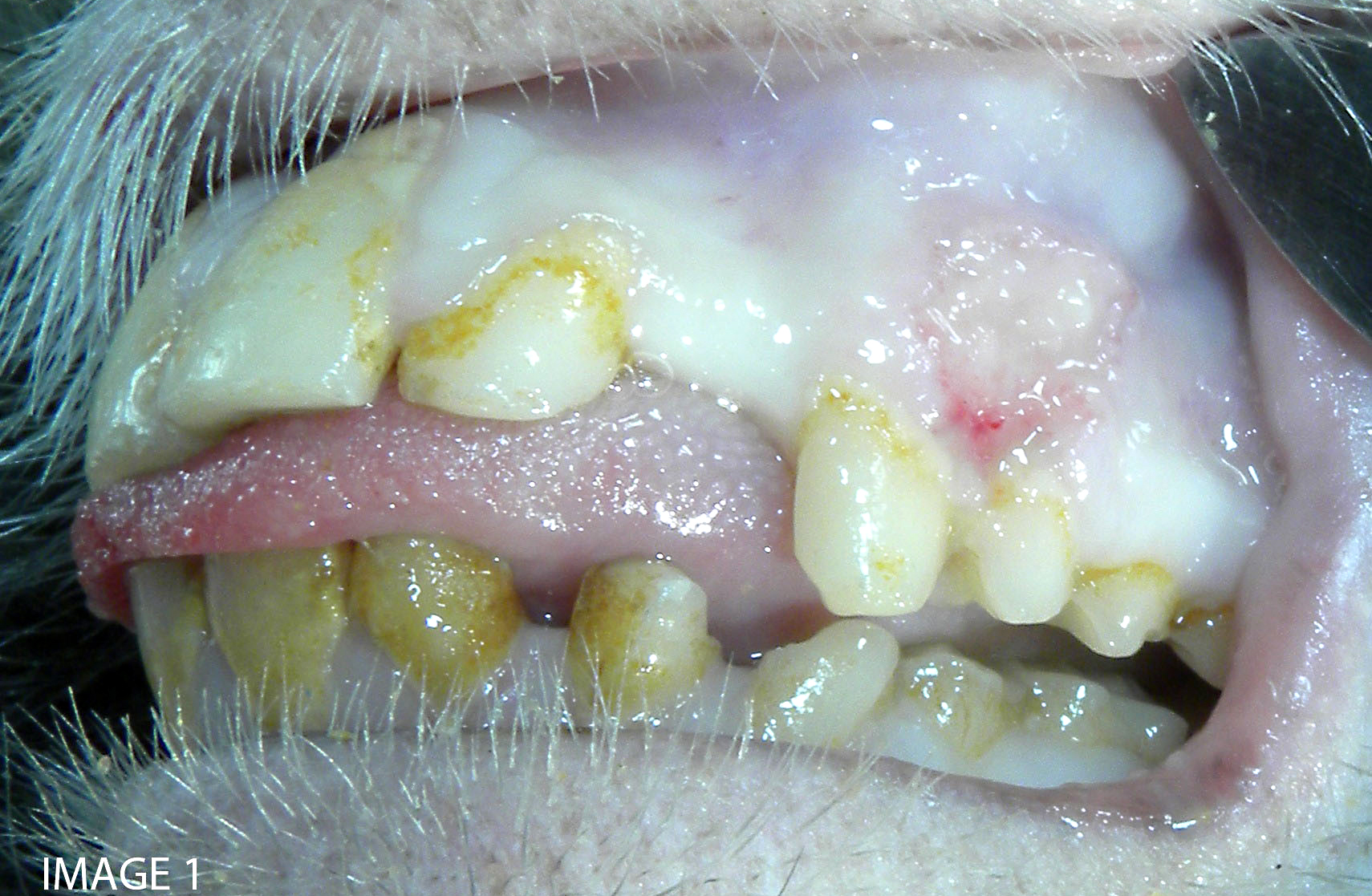
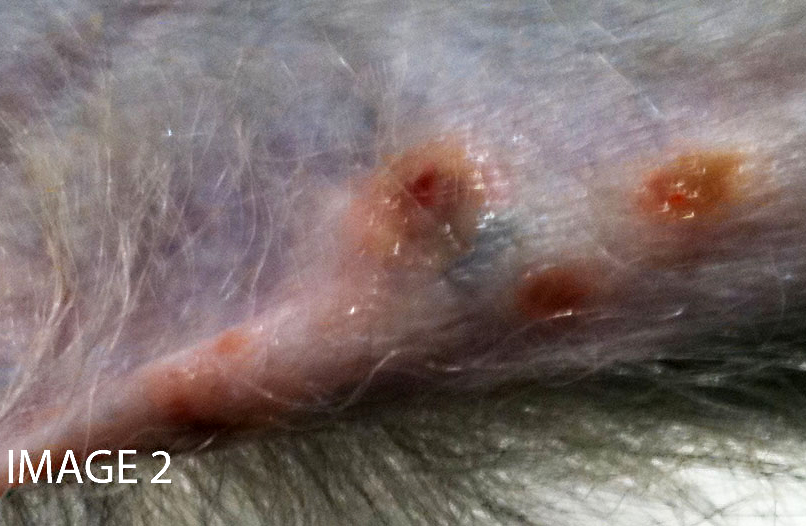
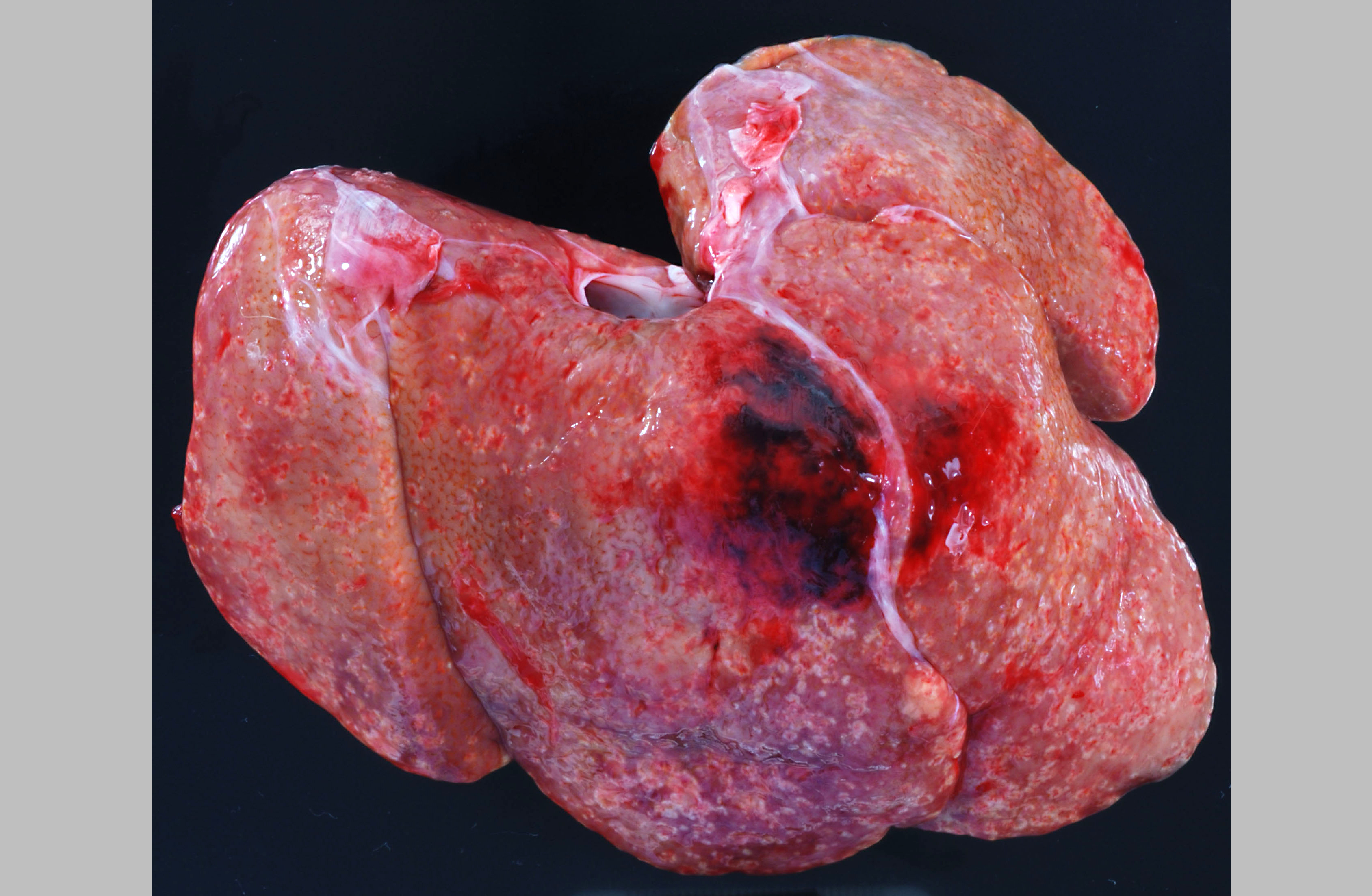
Approximately 150 cc of hemorrhagic fluid was present within the peritoneal cavity. The liver was pale, friable and moderately enlarged with rounded margins. The hepatic capsule was dull and irregular with random, multifocal to coalescing areas of necrosis interspersed among beaded yellow-brown foci arranged in a reticulate fashion. The necrotic foci were either miliary or exhibited target-like lesions with distinct, raised white rims and depressed red centers that measured up to 0.4 cm in greatest dimension. The spleen was pale, enlarged and pulpy with multiple, irregular foci of necrosis. The splenic margins were rounded and the capsule was smooth and taut. The kidneys were streaked and pale and bulge on sectioned surface. There were multifocal hemorrhages throughout the adrenal glands. Multiple cervical hemorrhages were present and the mucosa was overlain by a fibrinopurulent exudate.
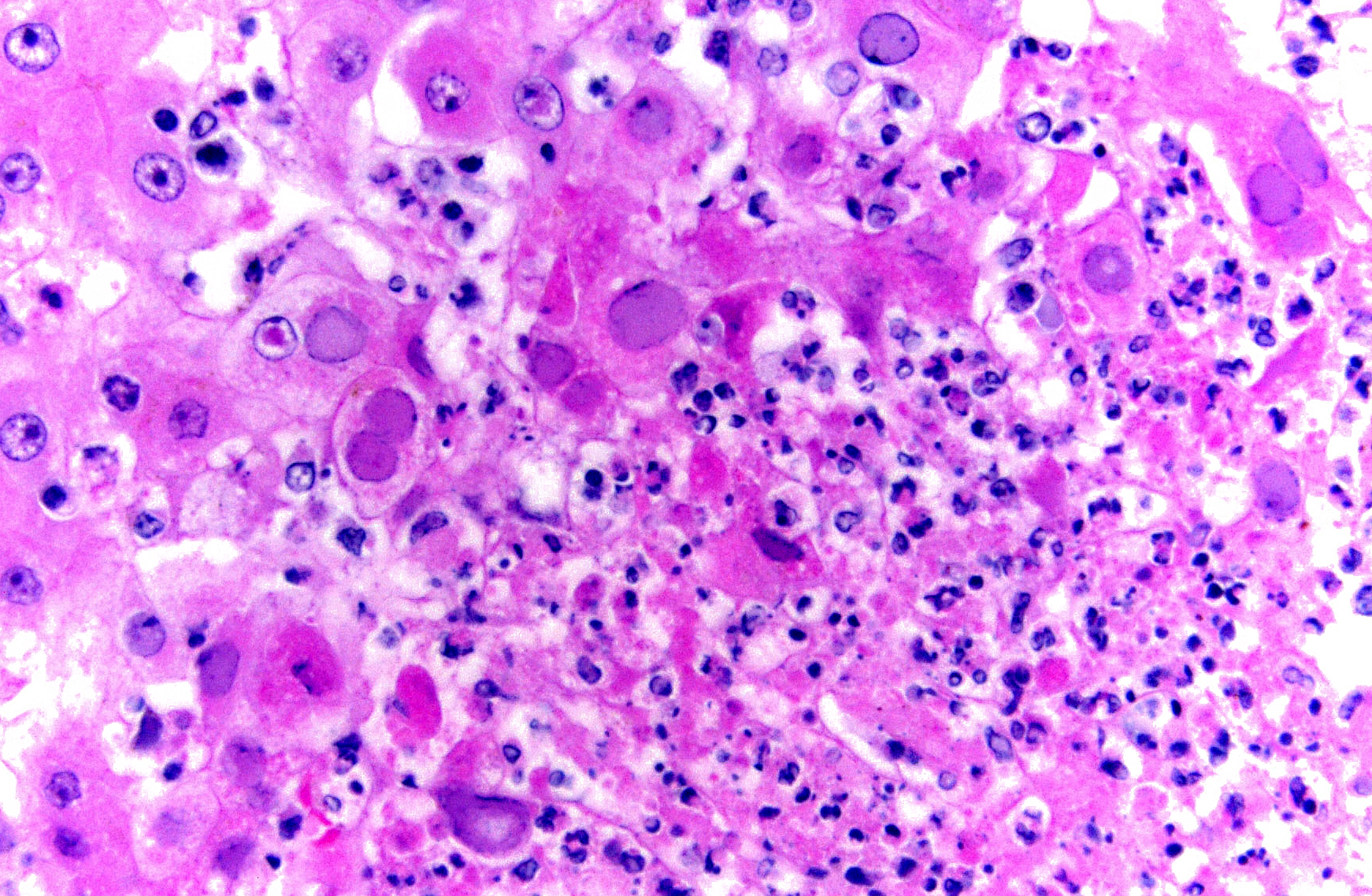
Histopathologic Description:
Morphologic Diagnosis:
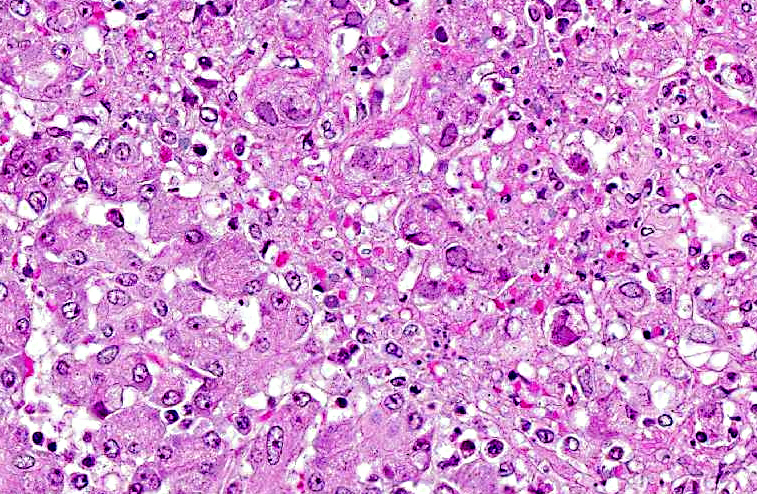
Microscopic findings of tissues not submitted: Neutrophilic fibrinonecrotizing inflammation affected the spleen, adrenal gland, liver, tonsil, cervix, esophagus, haired skin, gingiva, and lip. Most tissues contained intranuclear inclusion bodies and syncytia as described in the pancreas.
Lab Results:
Macacine herpesvirus 1 Testing
| Sample | Test | Result |
| Vaginal mucosal swab Cell | culture, PCR* | Positive |
| Oral mucosal swab | Cell culture, PCR* | Positive |
| Liver | Cell culture, PCR* | Positive |
| Spleen | Cell culture, PCR* | Negative |
| Serum, 6 years prior to death | IgG Antibody ELISA** | Negative |
| Serum, 5 weeks prior to death | IgG Antibody ELISA & Western blot* | Negative |
| Serum, 2 days prior to death | IgG Antibody ELISA | Positive |
| Serum, 2 days prior to death | IgG Antibody Western blot | Negative |
| Serum, 2 days prior to death | IgM Antibody Western blot | Positive |
**In-house laboratory
| Analyte (unit) | Value | Reference interval |
| ALT (IU/L) | 202 | 0 37 |
| ALP (IU/L) | 1887 | 30 120 |
| Total protein (g/dl) | 4.8 | 6.0 8.0 |
| Albumin (g/dl) | 1.3 | 3.0 4.1 |
| RBC (x106/μL) | 3.9 | 5.0 6.5 |
| PCV (%) | 21.1 | 35 45 |
| Platelets (x103/μL) | 112 | 330 650 |
| Leukocytes (x103/μL) | 5.0 | 6.00 14.00 |
| Neutrophils (x103/μL) | 2.45 | 2.40 11.20 |
| Bands (x103/μL) | 0.50 | 0 |
| Lymphocytes (x103/μL) | 2.35 | 1.50 7.00 |
| Monocytes (x103/μL) | 0.15 | 0 1.12 |
| Eosinophils (x103/μL) | 0 | 0 0.70 |
| Basophils (x103/μL) | 0 | 0 0.28 |
Condition:
Contributor Comment:
Swabs of the vaginal and oral mucous membranes and hepatic and splenic tissue samples were submitted to the National B Virus Resource Laboratory at Georgia State University for virus isolation. All samples were positive for McHV1. Serum collected five weeks prior to death was McHV1 IgG antibody negative and a second sample collected two days prior to death had detectable levels of IgG antibody on recombinant ELISA and IgM antibody on western blot. Seroconversion during experimental primary infection results in detectable levels of IgM six days post-infection and IgG 12 days post-infection.(9) IgM levels peak at 12 days, followed by rapid decline. In this case, prior negative tests, positive serum IgM and IgG antibodies, and the acute disease course suggest this was a primary infection.
McHV1 is an enveloped double stranded DNA virus, member of the family Herpesviridae, subfamily Alphaherpesvirinae, genus Simplexvirus. It is endemic in Asian macaques and has been isolated from rhesus (M. mulatta), cynomolgus (M. fascicularis), stump-tailed (M. arctoides), pigtail (M. nemestrina), Japanese (M. fuscata), bonnet (M. radiata), and Taiwanese (M. cyclopis) macaques.(8) It is genetically similar to Human herpesvirus 1 and 2, though phylogenetic analysis indicates it is more closely related to non-human primate α-herpesviruses Cercopithecine herpesvirus 2 (simian agent 8), and Papiine herpesvirus 2 (Herpesvirus papio 2).(6)
Transmission occurs horizontally with increasing seroprevalance coinciding with sexual maturity and typically approaching 80% in captive and wild adult cohorts.(6) Primary infection occurs when orogenital mucosa or open wounds are inoculated with virus-contaminated oral or genital fluid, blood, urine, or feces. McHV1 initially replicates in the epithelium at the site of inoculation and may result in vesicular lesions in the mucosae and skin of the mucocutaneous junctions of the oral cavity, genitals, and conjunctiva, though infection is typically asymptomatic. Virus is transported along axons of sensory neurons to ganglia, and subsequent cessation of replication results in latent infection. Virus reactivation and anterograde axonal transport to epithelium with recrudescence and viral particle shedding can occur in the absence of clinical disease or with similar vesicular lesions. Virus shedding is reportedly more prevalent during the breeding season.
Similar to other members of the Alphaherpesvirinae, fatal cross-species transmission can occur. As such, McHV1 is an important zoonotic disease for humans interacting with macaque species. Exposure to infected bodily fluids via mucosa membranes, open skin, and penetrating wounds can result in infection. Infection may produce vesicular lesions at the site of exposure, flu-like symptoms of fatigue, body aches, and fever, and fatal encephalitis has occurred in greater than 70% of documented cases. Excellent reviews detailing prevention and post-exposure treatment are available.(4,6,7)
Spontaneous disseminated McHV1 infection in macaque species that results in death or euthanasia is infrequent, and animals that develop any McHV1 lesions are typically culled rather than treated clinically due to the zoonotic risk to personnel. Primary and latent reactivation resulting in systemic infection has been described in rhesus (M. mulatta), cynomolgus (M. fascicularis), bonnet (M. radiata), and pigtail (M. nemestrina) macaques and virus has been isolated from brain, oral cavity, esophagus, liver, adrenal gland, pancreas, and skin.(1,2,3,5,11,12) Latent reactivation is typically associated with immunosuppression by comorbid disease, including dystocia and simian retrovirus type D infection, social stress, and with chronic administration of immunosuppressive drugs.(1,2,3,11,12) In this case, serologic testing suggests this was a na+â-»ve animal that contracted McHV1 from another animal in the cohort. Review of the clinical history and experimental treatments did not reveal a potential source of immunosuppression. Testing for simian type D retrovirus was done multiple times over a sixyear period with consistently negative results (blood, cell culture; serum, IF membrane antibody).
JPC Diagnosis:
Conference Comment:
Alphaherpesvirinae is subdivided into four genera: Simplexvirus, Varicelovirus, Mardivirus, and Iltovirus.(10) Most alphaherpesviruses grow rapidly, lyse infected cells and establish latent infections in sensory ganglia.
Members of subfamily Betaherpesvirinae are also divided into four genera: Cytomegalovirus, Muromegalovirus, Proboscivirus and Roseolovirus. Betaherpesviruses are generally characterized by a slow replication cycle and delayed cell lysis; they often remain latent in secretory glands, the kidneys, and lymphoreticular tissue.(10)
Gammaherpesviruses are generally lymphotropic and reside latent in lymphocytes. Some gammaherpesviruses have been linked to oncogenic transformation of lymphocytes such as Epstein-Barr virus resulting in Burkitts lymphoma. Although gammaherpesviruses of ruminants and nonhuman primates generally do not cause significant disease in their immunocompetent natural hosts, some may cause severe lymphproliferative disease in alternate hosts.(10)
Alphaherpesviruses of importance in veterinary medicine(10):
| Bovine Herpesvirus 1 | Infectious Bovine Rhinotracheitis and Infectious Pustular Vulvovaginitis Viruses |
| Bovine Herpesvirus 2 | Mammillitis/ Psuedo-Lumpy Skin Disease Virus |
| Bovine Herpesvirus 5 | Bovine Encephalitis Virus |
| Canid Herpesvirus | Systemic hemorrhagic disease in pups <4 weeks old |
| Caprine Herpesvirus | Abortion; Infectious pustular vulvovaginitis |
| Macacine Herpesvirus 1 | B Virus disease of macaques (formerly Cercopithecine Herpesvirus 1) |
| Herpes simplex 1 | Severe generalized disease with high mortality in New World primates |
| Cercopithecine Herpesvirus 9 | Simian Varicella Virus |
| Equid Herpesvirus 1 | Equine Abortion Virus |
| Equid Herpesvirus 3 | Equine Coital Exanthema Virus |
| Equid Herpesvirus 4 | Equine Rhinopneumonitis Virus |
| Felid Herpesvirus 1 | Feline Rhinotracheitis Virus |
| Gallid Herpesvirus 1 | Avian Infectious Laryngotracheitis Virus |
| Gallid Herpesvirus 2 | Mareks Disease Virus |
| Suid Herpesvirus 1 | Pseudorabies or Aujeszkys Disease Virus |
Betaherpesviruses of importance in veterinary medicine(10):
| Murid Herpesvirus 1 | Mouse Cytomegalovirus |
| Murid Herpesvirus 2 | Rat Cytomegalovirus |
| Caviid Herpesvirus 2 | Guinea Pig Cytomegalovirus |
| Rhesus Cytomegalovirus | *Many Old and New World non-human primates have their own cytomegaloviruses |
| Elephantid Herpesvirus | Endotheliotropic Elephant Herpesvirus |
| Suid Herpesvirus 2 | Porcine Cytomegalovirus |
Gammaherpesviruses of importance in veterinary medicine(10):
| Alcelaphine Herpesvirus 1 | Malignant Catarrhal Fever |
| Ovine Herpesvirus 2 | Malignant Catarrhal Fever |
| Equid Herpesvirus 5 | Multinodular Pulmonary Fibrosis |
| Saimiriine Herpesvirus 2 (Herpesvirus saimiri) and Herpesvirus ateles | T lymphocytotropic virus that causes subclinical latent infections in squirrel monkeys (H. saimiri) and spider monkeys (H. ateles), but rapid and fatal lymphoproliferative disease in New World monkeys (marmosets, tamarins, owl monkeys) |
| Retroperitoneal Fibromatosis Herpesvirus and Rhesus Rhadinovirus | Retroperitoneal fibromatosis and B-cell lymphomas in retroviral-infected Rhesusmacaques |
Unassigned members of Herpesviridae of importance in veterinary medicine10:
| Anatid Herpesvirus 1 | Duck Viral Enteritis Virus; Duck Plague Virus |
References:
2. Carlson CS, OSullivan MG, Jayo MJ et al. Fatal disseminated Cercopithecine herpesvirus 1 (Herpes B) infection in cynomolgus monkeys (Macaca fascicularis). Vet Pathol. 1997;34:405- 414.
3. Chellman GJ, Lukas VS, Eugui EM, Altera KP, Almquist SJ, Hilliard JK. Activation of B virus (Herpesvirus simiae) in chronically immunosuppressed cynomolgus monkeys. Lab Anim Sci. 1992;42(2):146-151.
4. Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE. Recommendations for prevention and therapy for exposure to B virus (Cercopithecine herpesvirus 1). Clin Infect Dis. 2002;35:1101-1203.
5. Daniel MD, Garcia FG, Melendez LV, Hunt RD, OConnor J, Silva D. Multiple Herpesvirus simiae isolation from a rhesus monkey which died of cerebral infarction. Lab Anim Sci. 1975;25(3):303-308.
6. Elmore D, Eberle R. Monkey B virus (Cercopithecine herpesvirus 1). Comp Med. 2008;58(1):11-22.
7. Estep RD, Messaoudi I, Wong SW. Simian herpesviruses and their risk to humans. Vaccine. 2010;28S:B78-B84.
8. Huff JL, Barry PA. B-virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg Infect Dis. 2003;9(2):246-250.
9. Lees DN, Baskerville A, Cropper LM, Brown DW. Herpesvirus simiae (B virus) antibody response and virus shedding in experimental primary infection of cynomolgus monkeys. Lab Anim Sci. 1991;41:360-364.
10. Maclachlan NJ, Dubovi EJ, Herpesvirales. In: Fenner's Veterinary Virology. 4th ed. London, UK: Elsevier;2011:179-201.
11. Scharf BA, Wan CH, Bluth M, et al. Lethargy, ulcers, bronchopneumonia and death in two aged female bonnet macaques presumed to be caused by Cercopithecine herpes virus 1. J Med Primatol. 2008;37(Suppl1):60-64.
12. Simon MA, Daniel MD, Lee-Parritz D, King NW, Ringler DJ. Disseminated B virus infection in a cynomolgus monkey. Lab Anim Sci. 1993;43(6):545-550.