Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 20
25 March, 2020
CASE III: Blank label (JPC 4117883).
Signalment: 1-year-old, Aberdeen Angus steer, Bos taurus
History: Found dead in pen.
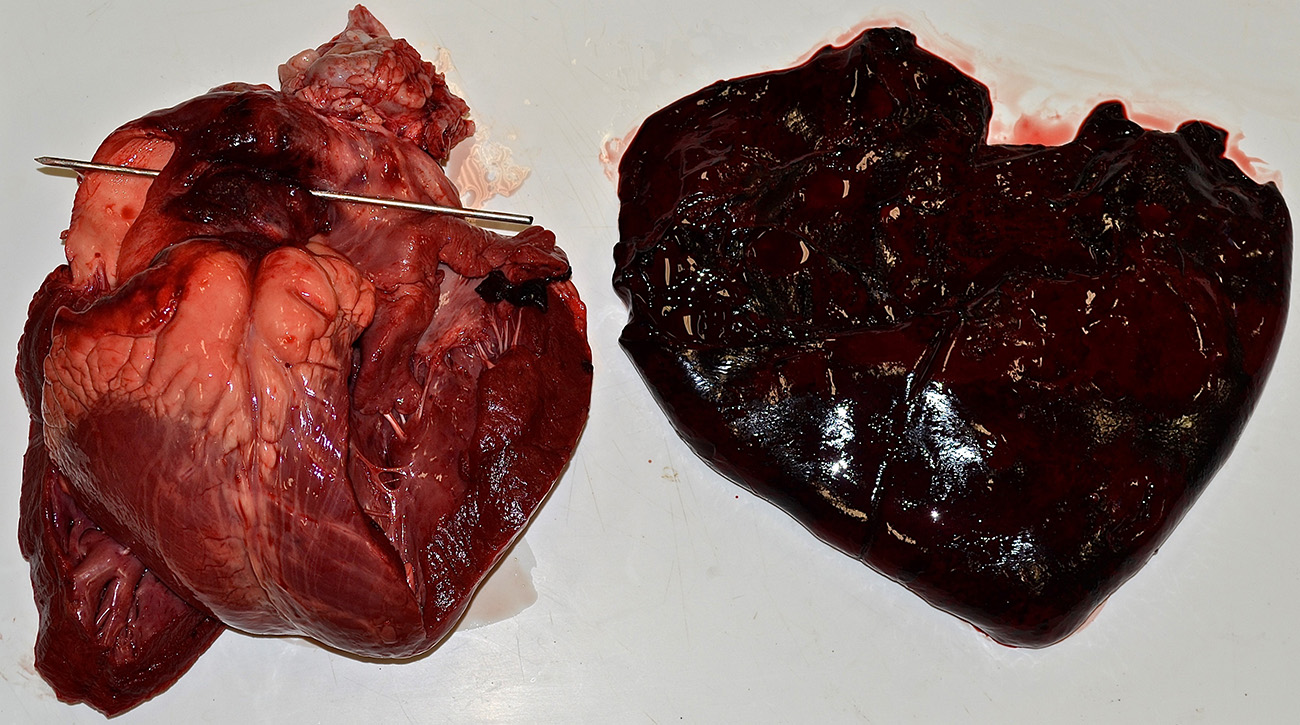
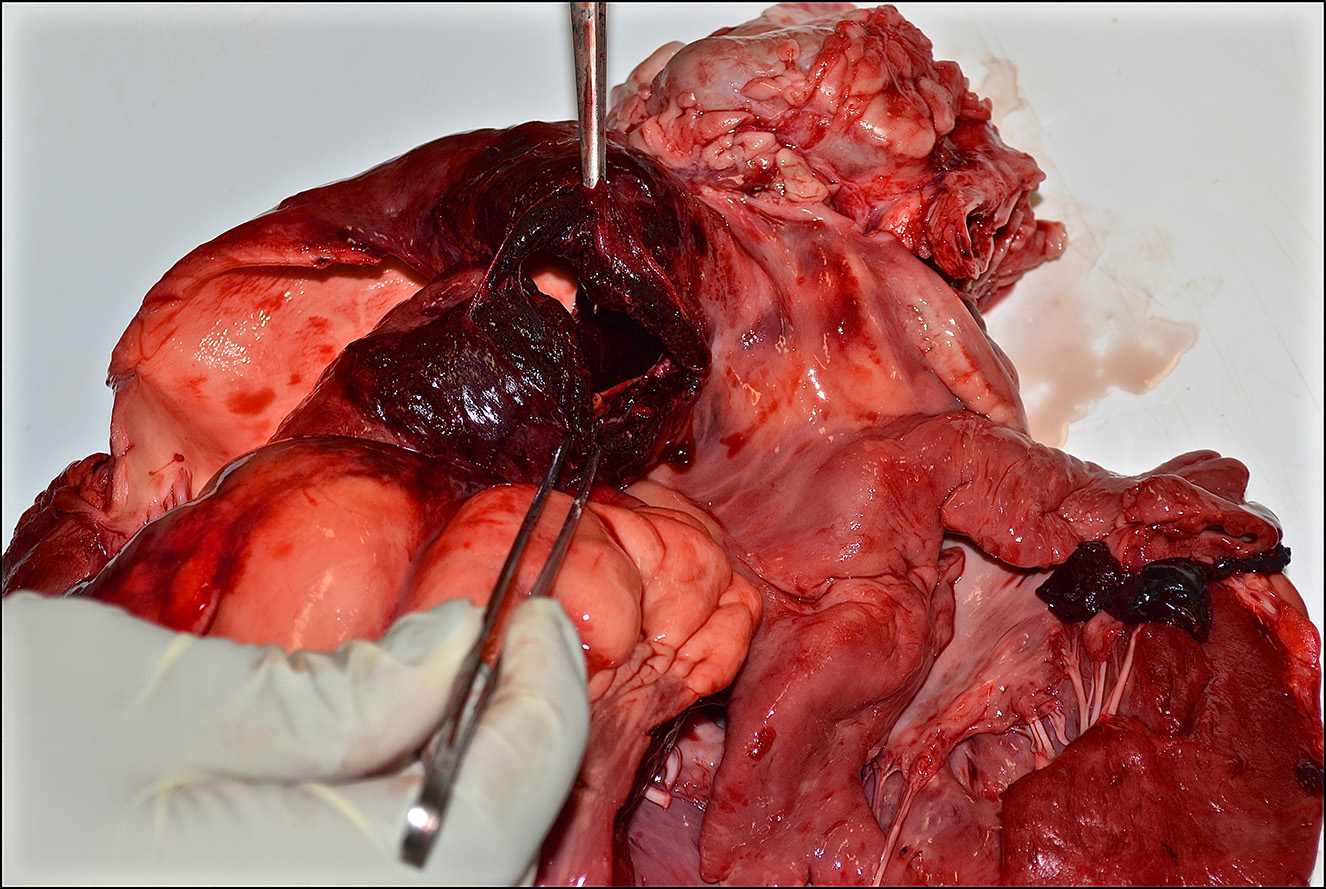
Gross Pathology: Presented for postmortem examination was a 1-year-old Black Angus steer in adequate body condition (BCS 2.5 out of 5) with minimal postmortem autolysis. A mild amount of edema expanded the subcutaneous fascia of the ventral thorax and mandible. Markedly distending the pericardial sac and enveloping the heart was a large ~2L coagulum of blood (Figure 1). The wall of the right ventricle was markedly thickened and firm, with a 1:1 ratio of right to left ventricular free walls. The pulmonary artery was severely dilated and there was a 4 x 2 cm acute full thickness tear at the base of the pulmonic outflow track (Figure 2). The intimal surface of the pulmonary artery was irregularly roughened and granular and the tear had hemorrhagic borders. The liver was slightly enlarged with rounded borders and was slightly firm in texture on cut section. The remainder of the post mortem examination was within normal limits.
1. Heart/pulmonary artery: Severe right ventricular concentric hypertrophy with pulmonary artery aneurysm, acute rupture, and hemopericardium with cardiac tamponade.
2. Liver: Moderate chronic passive congestion.
Laboratory results:
Bacteriology - Aerobic culture, lung
No significant growth
Molecular diagnostics - lung
Mycoplasma PCR: Not detected
Bovine respiratory syncytial virus (BRSV) real-time PCR: Not detected
Bovine herpesvirus-1 (IBR) real-time PCR: Not detected
Bovine viral diarrhea virus (BVDV) real-time PCR: Not detected
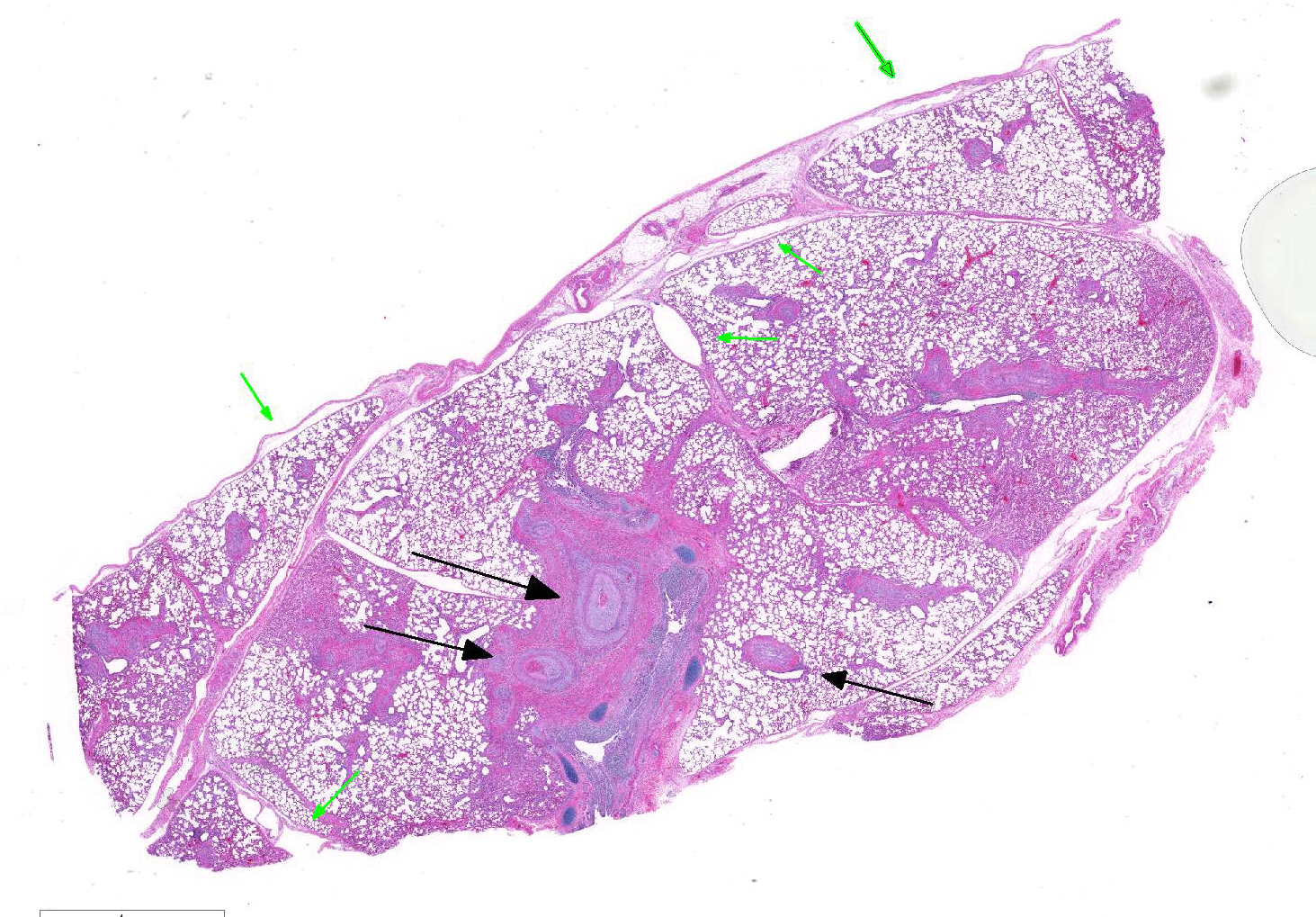
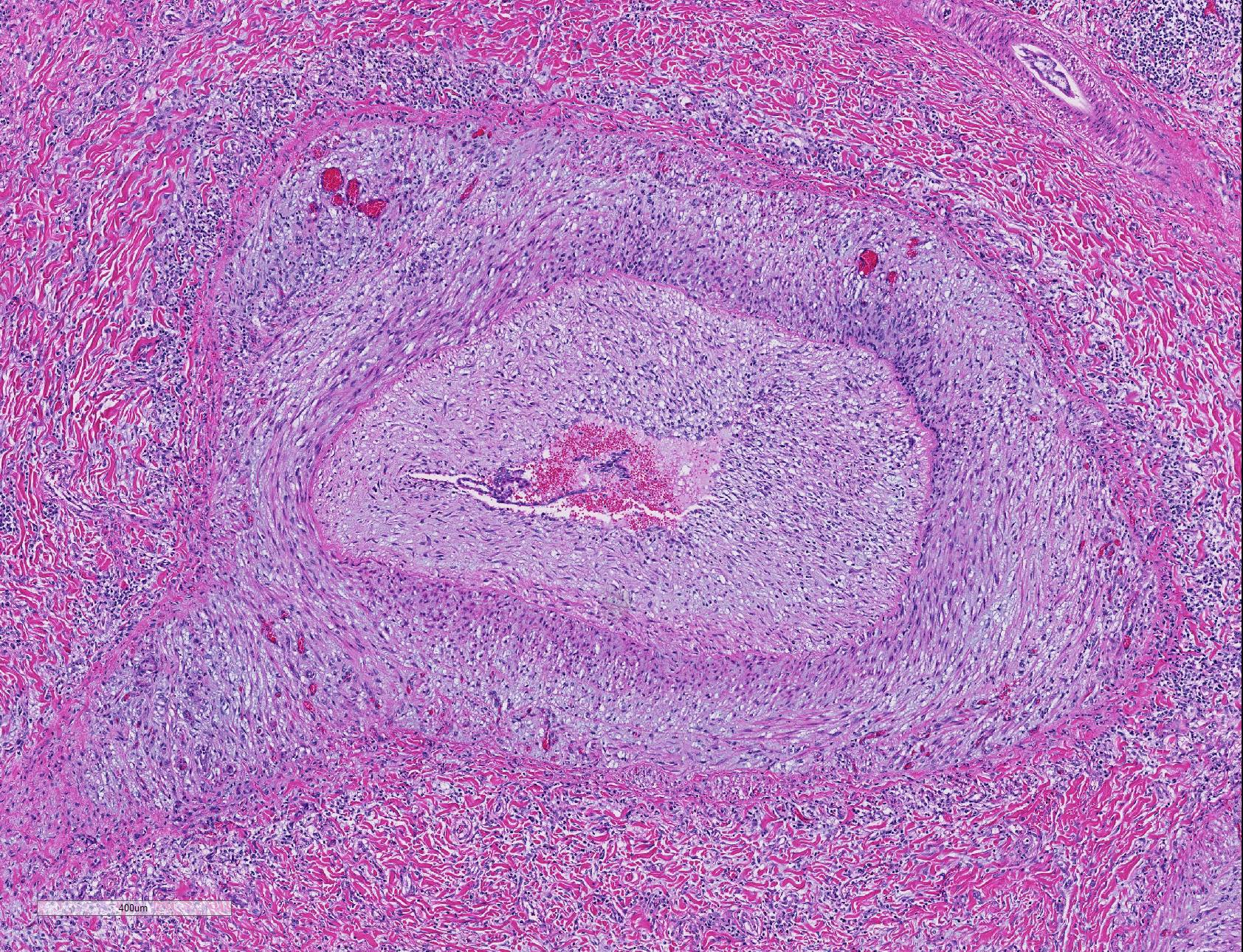
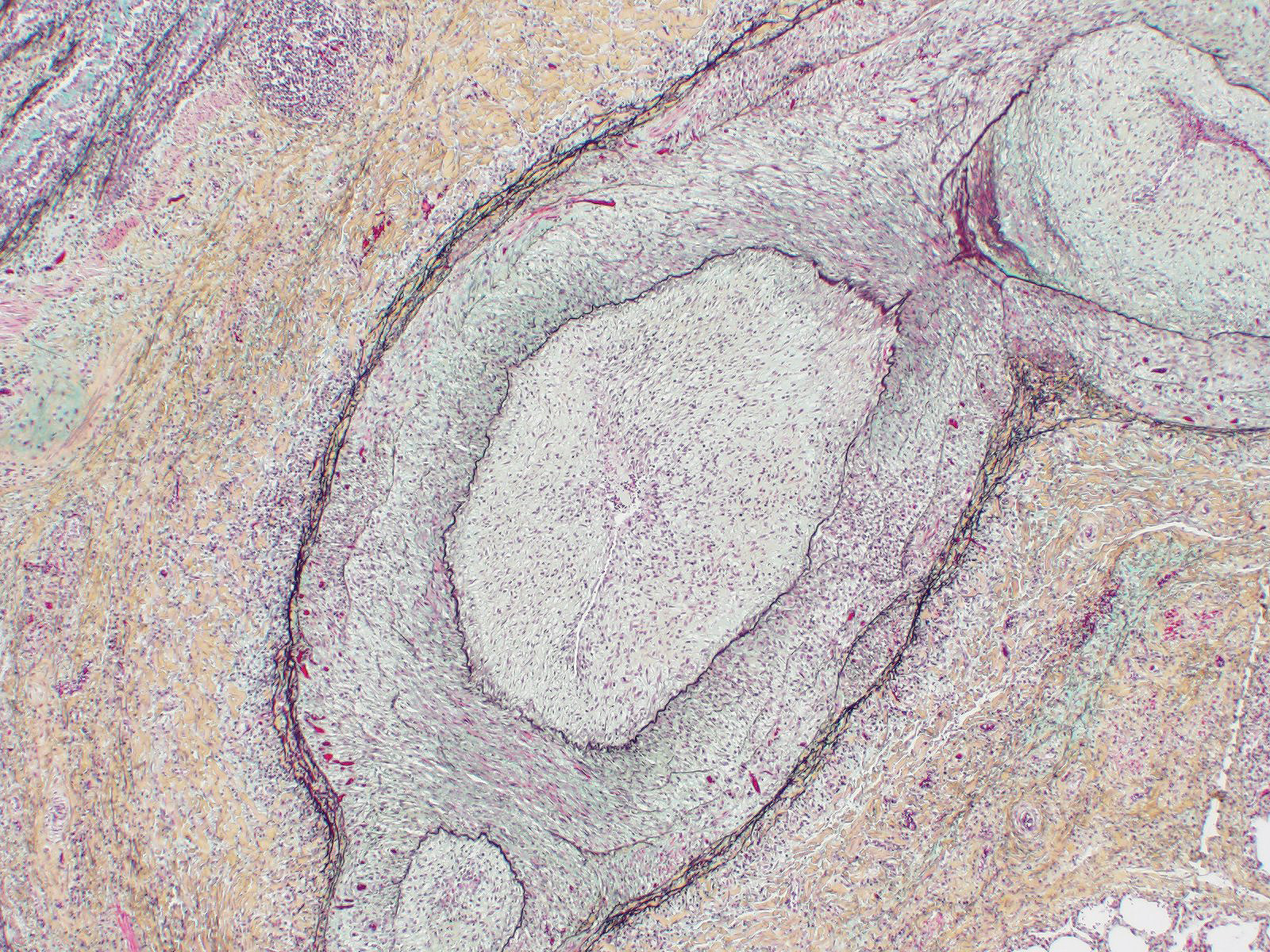
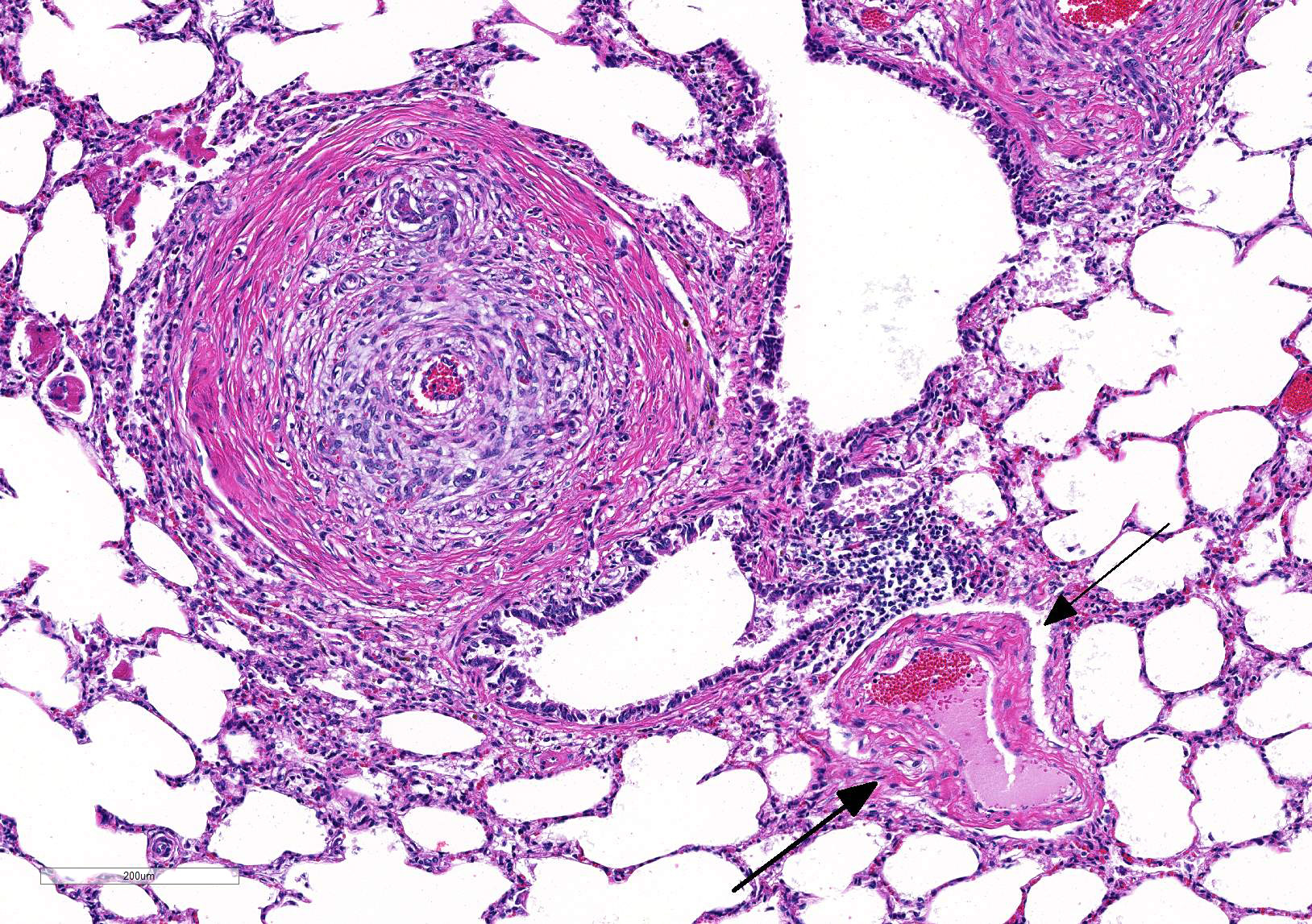
Microscopic Description: Lung: Diffusely throughout sections examined, the vascular lumina of small to large caliber muscular arteries, and to a lesser extent intraparenchymal pulmonary veins, are diminished due to marked expansion of the tunica intima and media by hypertrophic and hyperplastic smooth muscle and fibroplasia. Severely affected vessels are also segmentally to circumferentially expanded by poorly organized and edematous adventitial fibrosis which supports multiple cross sections of proliferative capillary profiles (complex plexiform arteriopathy/vasa vasorum hypertrophy). Adjacent alveolar spaces are occasionally compressed and collapsed. Vessels are internally lined by endothelial cells which are plump and reactive. Rare vessels are partially to completely occluded by organized thrombi. The tunica intimal and medial hypertrophy of both arteries and (to a lesser extent) veins was confirmed on a Van Gieson stain.
There are multifocal regions of mild alveolar interstitial necrosis. In these areas alveolar septate are lined by hyalinized webs of fibrin. In other locations alveolar septae are lined by plump hyperplastic type II pneumocytes. Alveolar spaces are multifocally expanded by fibrinous and edematous exudates which support scant numbers of neutrophils and plump foamy alveolar macrophages. The pleural surface and interlobular septae are mildly expanded by edema.
Contributor Morphologic Diagnosis:
1. Lungs, pulmonary arteries: Severe diffuse chronic arterial mural hypertrophy and hyperplasia with adventitial fibrosis and vasa vasorum hypertrophy (plexiform arteriopathy).
2. Lungs, pulmonary veins: Multifocal mild segmental mural hypertrophy.
Contributor Comment: The most significant gross lesion detected on post mortem examination was severe right ventricular concentric hypertrophy with pulmonary artery aneurysm, rupture, and hemopericardium. Histologic lesions within the lungs are consistent with high mountain disease, also known as brisket disease due to typical presentation with submandibular, cervical, and cranioventral thoracic subcutaneous edema.
Brisket disease was clinically recognized in Colorado in the early 1900?s and was initially reported by Glover and Newsom in the Colorado Agriculture Experimental Station bulletin in 1915 and 1917.2,3 In 1959 Alexander and Jensen reported that this condition is associated with right ventricular hypertrophy in both natural cases as well as in experimental cattle residing at high altitude.1 Chronic hypoxia and pulmonary hypertension were proposed as an underlying etiology.
In acute hypoxic conditions, such as encountered at high altitude (elevation > 1600m), pulmonary arteriolar vasoconstriction results in both pulmonary hypertension and vascular shunting.5 Chronicity results in remodeling of pulmonary arteries, including hypertrophy of the tunica intima, media, and adventitia, which further increases pulmonary resistance to flow. Neary et. al speculated that proximal remodeling of large pulmonary arteries (in additional to precapillary arterioles) may play a role in the pathogenesis of bovine pulmonary hypertension.8 Stenmark et al. introduced an ?outside in? hypothesis which postulates that vascular remodeling may be initiated by activated pro-inflammatory adventitial fibroblasts which mediate remodeling in adventitial, medial, and intimal layers and induce proliferation of the vasa vasorum.6,10 Long-standing pulmonary hypertension may result in cor pulmonale or right ventricular cardiac hypertrophy and eventually right-sided congestive heart failure.5
Pulmonary hypertension is detected in live cattle by direct measurement of pulmonary arterial pressure (PAP) via catheterization of the right ventricle. Routine pulmonary arterial pressure testing (PAP) was initiated in the 1960s and early studies concluded that the degree of pulmonary hypertension detected by the PAP measurement was directly related to the degree of histologic pulmonary arterial hypertrophy.5 Thus a pulmonary arterial pressure above 30 mmHg is considered diagnostic for pulmonary hypertension in cattle and it is recommended bulls with an elevated PAP be removed from breeding stock.
As demonstrated in this case by Verhoff-Van Gieson elastin staining, vascular lesions are not limited solely to pulmonary arteries but also rarely involve pulmonary veins. The degree of this lesion is mild in this case, but may be playing a perpetuating role in the underlying pathogenesis of this disease.
In addition to cattle, cardiac and pulmonary arterial remodeling was described in various zoo mammals housed at high altitudes at African Safari in Puebla, Mexico (elevation 2,100m) (8). Species included in this study were 10 maras (Dolichotis patagonum), 2 cotton-top tamarins (Saguinus oedipus oedipus), 2 capybaras (Hydrochaeris hydrochaeris), a Bennet?s wallaby (Macropus rufogriseus), a nilgai antelope (Boselaphus tragocamelus), and a scimitar-horned oryx (Oryx dammah).9
It is important to rule out other diseases that may mimic brisket disease such as hardware disease, cardiomyopathy, heart failure secondary to chronic pneumonia, diffuse pulmonary parenchymal disease such as pulmonary fibrosis, or disseminated pulmonary abscesses, parasitic pneumonia, pulmonary intoxications, and/or emphysema.7 In this case, the histologic changes consistent with a mild interstitial pneumonia retrospectively prompted a comprehensive bovine respiratory disease screen for case completeness; no underlying infectious etiologies were identified.
Given the history of this animal being raised along the Colorado Front Range (elevation ~1700m) combined with the gross and histologic diagnoses we conclude the constellation of these findings are consistent with bovine high altitude pulmonary hypertension with core pulmonale, pulmonary aneurysm and rupture.
Contributing Institution:
Colorado State University Veterinary Diagnostics Laboratories
200 West Lake Street
1644 Campus Delivery
Fort Collins, CO 80523-1644
http://csu-cvmbs.colostate.edu/vdl/Pages/default.aspx
JPC Diagnosis: Lung, pulmonary arterioles: Smooth muscle hypertrophy and hyperplasia, eccentric, diffuse, severe, with mural fibroplasia, venous fibroplasia, and multifocal septal necrosis.
JPC Comment: The World Health Organization has classified six different types of pulmonary hypertension: pulmonary arterial hypertension, pulmonary veno-occlusive disease, hypertension resulting from left heart disease, hypertension with pulmonary disease or hypoxemia (which includes chronic forms of altitude disease), chronic thromboembolic pulmonary disease, and pulmonary hypertension with unclear multifactorial mechanisms.11
In humans, altitude disease (?mountain sickness?) takes both
an acute and chronic form. Acute exposure to hypoxic conditions at high
altitude (2500 ft above sea level) manifests within 6-12 hours, characterized
by headache, loss of appetite, vomiting, dizziness, and fatigue. Two theories
as to its pathogenesis predominate: a) acute cerebral hyperperfusion resulting
from impeded autoregulation of cerebral blood vessels and increased levels of
VEG-F and circulating radicals, and b) inhibition of astrocytic Na-K ATPase and
resulting edema and irritation of sensory trigeminal nerve fibers. If
unattended, especially in additional altitude is attained, life-threatening
cerebral or pulmonary edema may result.4
Chronic hypoxia resulting from high altitude in humans results in significant
remodeling of small- and medium caliber pulmonary arterioles. The remodeling
is the result of increased proliferation, decreased apoptosis, and migration of
a population of less well-differentiated pulmonary artery smooth muscle cells.
In addition, a mural population of fibroblasts may differentiate into a smooth
muscle phenotype, while maintaining the ability to secrete matrix proteins.
The end result of both proliferations results in effacement of the tunica
intima and expansion of all layers of the arterial wall by smooth muscle
hyperplasia, disarray, and fibrosis. In affected lungs, previously
non-muscularized precapillary vessels may develop a muscular wall. However,
all of these changes are reversible after prolonged re-exposure to normal
oxygen levels.4
Plexiform lesions are not seen in remodeling of pulmonary arteries in chronic
hypoxia in humans, and are associated with the most severe group of diseases
resulting in pulmonary hypertension, those in WHO group 1, pulmonary arterial
hypertension. This group includes the idiopathic and congenital forms of
pulmonary arterial hypertension, which results in severe, non-reversible
remodeling changes of pulmonary arterial remodeling, to include fibrinoid
arteritis and plexiform lesion development.11 An excellent example
of these changes may be seen in WSC 25, Case 4 of 2018-2019, in a West Highland
White Terrier, a breed in which several cases of idiopathic pulmonary hypertension
similar to that seen in humans have been documented.
Several animal models for pulmonary hypertension exist.
Chronic exposure of rats and mice to diminished levels of oxygen in normo- and
hypobaric settings have been utilized for years and produce predictable and
reproducible results, depending on the strain of rodent. Fawn-hood rats appear
to produce the most dramatic tissue changes. Oral administration of
monocrotaline, a toxic pyrrollizzidine alkaloid derived from Crotalus
spectabilis also produces arterial changes consistent with pulmonary
hypertension in rats dosed orally. While the precise mechanism is unknown, the
prevailing hypothesis suggests direct endothelial damage to pulmonary
arterioles. In addition, several genetically engineered mouse strains,
including a BMPR2 knock out model (a genetic mutation seen in humans with
severe forms of pulmonary arterial hypertension) have been developed.11
References:
1. Alexander AF, Jensen R. Gross Changes in Cattle with High Mountain (Brisket) Disease and in Experimental Cattle Maintained at High Altitudes.
Am J Vet Res 20:680?689. 1959.
2. Glover GH, Newsom IE. Brisket Disease (Dropsy of
High Altitude). The Agriculture Experiment Station of the Colorado
Agricultural College Bulletin No. 204. January, 1915.
3. Glover GH, Newsom IE. Brisket Disease. The Agriculture Experiment Station of the Colorado Agricultural College Bulletin No. 204. May 1917.
4. Grimminger J, Richer M, Tello K, Sommer N,
Hening G, Ghofrani HA. Thin air resulting in high pressure: Mountain sickness
and hypoxia-induced pulmonary hypertension. Can Resp J
2017;https://doi.org/10.1155/2017/8381653.
5. Holt TN, Callan RJ. Pulmonary Arterial Pressure
Testing for High Mountain Disease in Cattle. Vet Clin North Am - Food Anim
Pract. 2007.
6. Li M, Riddle SR, Frid MG, et al. Emergence of
Fibroblasts with a Proinflammatory Epigenetically Altered Phenotype in Severe
Hypoxic Pulmonary Hypertension. J Immunol. 2011.
7. Malherbe CR, Marquard J, Legg DE, Cammack KM,
O?Toole D. Right ventricular hypertrophy with heart failure in Holstein heifers
at elevation of 1,600 meters. J Vet Diagn Invest. 2012.
8. Neary JM, Gould DH, Garry FB, Knight AP, Dargatz
DA, Holt TN. An investigation into beef calf mortality on five high-altitude
ranches that selected sires with low pulmonary arterial pressures for over 20
years. J Vet Diagnostic Investig. 2013.
9. Para A, Garner MM, Dipl ACVP. Pulmonary Arterial
Disease Associated With Right-Sided Cardiac Hypertrophy and Congestive Heart
Failure in Zoo Mammals Housed At 2,100 M Above Sea. J zoo Wildl Med.
2015.
10. Stenmark KR, Frid MG, Yeager M, et al. Targeting the Adventitial Microenvironment in Pulmonary Hypertension: A Potential Approach to Therapy that Considers Epigenetic Change. Pulm Circ. 2012.
11. Stenmark KR, Mcyrick B, Galie N, Mooi WJ, McMurtyr IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. AmJ Phiol Lung Cell Mol Physiol 2009; 197: L1013-1032.