Signalment:
Gross Description:
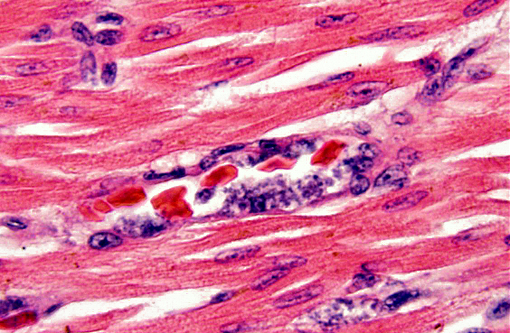
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Avian malaria associated with Plasmodium sp. is a major cause of avian morbidity and mortality in zoos worldwide, most notably captive penguin populations. Several Plasmodium sp. have been implicated as causative agents of avian malaria in captive penguins, including P. relictum, P. enlongatum, P. tejerai, and P. juxtanuclear.(2,4,8,11,12) Plasmodium sp. have a complex life cycle involving both asexual and sexual reproductive stages, which take place in the host and vector. When an infected female mosquito takes a blood meal, sporozoites are released into the hosts peripheral circulation. The sporozoites then invade cells of the reticuloendothelial system where they multiply and grow within parasitophorous vacuoles to produce schizonts, which can contain up to 10,000 30, 000 merozites (in the case of Plasmodium falciparum). Most commonly this development is in the liver, but they will also develop in other tissues such as the kidney, lungs, CNS, spleen, and heart. The schizonts produce thousands of merozoites, which are released into host cell-derived merosomes that are protected from host immunity. At this point individual merozoites are released into the circulation and infect erythrocytes. In the erythrocyte they develop into trophozoites (the feeding or ring stage).While feeding on hemoglobin they release hemoglobin pigments (hemozoin) which are a by-product of hemoglobin metabolism and a feature of the erythrocyte life cycle stage that can be appreciated on histopathology of the liver and lungs. Trophozoites then develop into a schizonts containing between 8-32 merozoites, which once released into the circulation can reinfect more erythrocytes recapitulating this stage of the infectious life cycle. After several cycles of invading erythrocytes, some of the merozoites transform into microgametocytes and macrogametocytes. Once a mosquito ingests erythrocytes containing these gametes, they further develop and fuse forming oocysts that yield the infective sporozoites.(1,6,7,10)
The extra-erythrocytic pathway of replication predominates in penguins, explaining the generally low levels of parasitemia seen on blood smears.(4,11,12) Previous studies demonstrated the utility of leukocyte counts as another predictive factor of parasitism to combine with blood smear evaluation. In cases with detectable parasitemia, the severity of leukocytosis was often a predictive factor in disease severity.(11) In this animal, the relative leukocyte count was greater than 75,000 WBC/uL. Immune suppression associated with stress, other infectious agents, or immaturity have been consistently demonstrated to be a contributing factor in Plasmodium disease progression in captive penguins.(3) Penguin mortality is closely associated with the presence of disseminated extra-erythrocytic replication, including in the liver, lung, heart, kidney, and CNS.(4,8,11)
This penguin came from a colony previously known to be infected with both Plasmodium relictum and P. elongatum. Historically, disease surveillance of this colony via blood smear analysis has often yielded low to undetectable blood parasite levels, yet, necropsy of a small numbers of these animals over a 15-year period reveal that some of the animals suffered from severe multi-organ disease attributed to extra-erythrocytic replication of Plasmodium sp. Similar to previous cases, intra-endothelial protozoa with morphology consistent with Plasmodium sp. schizonts were noted in the liver, lung, spleen, kidney, meninges, brain, and heart. Repeated episodes of seizures in this animal are attributed to the development of meningoencephalitis. Interestingly, focal arterial thrombosis was noted in several sections of the heart from this animal. Coagulopathies in humans infected with Plasmodium falciparum are thought to be associated with the ability of parasitized erythrocytes to activate the coagulation cascade through recognition of numerous receptors and pathways.(5) Based on this finding it is a possibility that a similar phenomenon may exist with avian malaria.
JPC Diagnosis:
Conference Comment:
In the penguin alone, schizonts, merozoites and gametocytes may be detected simultaneosly. Schizonts are smaller than the host nucleus and there is little cytoplasm present. The merozoite stage is directly adjacent to the erythrocyte nucleus, also with little or no cytoplasm. Gametocytes are variably shaped, from irregular to spherical, and occasionally displace the host cell nucleus.(8)
References:
1. Bermudez AJ. Miscellaneous and sporadic protozoal infections. In: Calnek BW, ed. Diseases of Poultry. 11th ed. Ames, Iowa: Iowa State Press;2003:1010-1015.
2. Bueno MG, Lopez RP, de Menezes RM, Costa-Nascimento Mde J, Lima GF, et al. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from S+â-úo Paulo Zoo, Brazil. Vet Parasitol. 2010;11:173(1-2):123-7.
3. Cranfield MR, Graczyk TK, Beall FB, Ialeggio DM, Shaw ML, Skjoldager ML. Subclinical avian malaria infections in African black-footed penguins (Spheniscus demersus) and induction of parasite recrudescence. J Wildl Dis. 1994;30(3):372-6.
4. Fix AS, Waterhouse C, Greiner EC, Stoskopf MK. Plasmodium relictum as a cause of avian malaria in wild-caught magellanic penguins (Spheniscus magellanicus). J Wildl Dis. 1988;24(4):610-9.
5. Francischetti IMB, Seydel KB, Montiero RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15(2):81-107.
6. Gardiner CH, Fayer R, Dubey JP. An Atlas of Protozoan Parasites in Animal Tissues. 2nd ed. Washingtn, DC: Armed Forces Institute of Pathology; 1998:65-66.
7. Greiner EC, Ritchie BW. Parasites. In: Ritchie BW, Harrison GJ, Harrison LR , eds. Avian Medicine: Principles and Application. Lake Worth, FL; Wingers Publishing, Inc. 1994:1019-1021.
8. Grim KC, Van der Merwe E, Sullivan M, Parsons N, McCutchan TF, Cranfield M. Plasmodium Juxtanucleare associated with mortality in black-footed penguins (Spheniscus demersus) admitted to a rehabilitation center. J Zoo Wildl Med. 2003;34(3):250-5.
9. Jones TC, Hunt RD, King NW. Veterinary Pathology. 6th ed. Baltimore, MD: Williams and Wilkins; 1996:590-593.
10. Olivier S, Mota M, Matuschewski K, Prud+â-¬ncio M. Interactions of the malaria parasite with its host. Current opinion in microbiology. Volume 11. 2008;(4):352359.
11. Stoskopf MK, Beier J. Avian malaria in African Black-Footed Penguins. JAVMA. 1972;175(9):944-7.
12. Vanstreels RE, Kolesnikovas CK, Sandri S, Silveira P, Belo NO, Ferreira Junior FC, et al. Outbreak of avian malaria associated to multiple species of Plasmodium in magellanic penguins undergoing rehabilitation in southern Brazil. PLoS One. 2014;15;9(4):e94994.