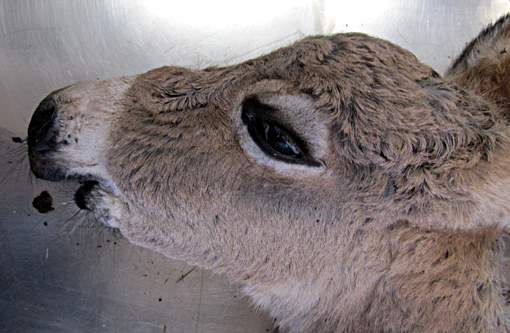
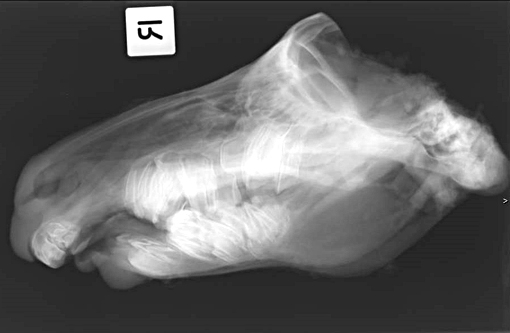
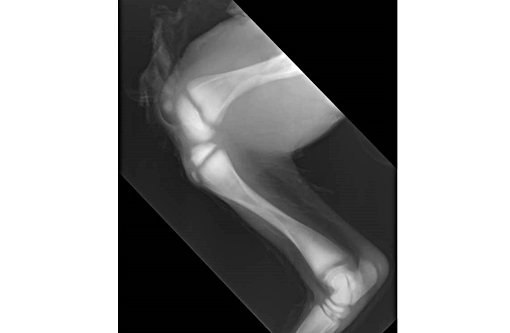
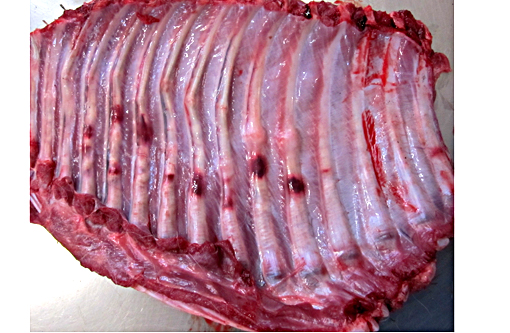
Signalment:
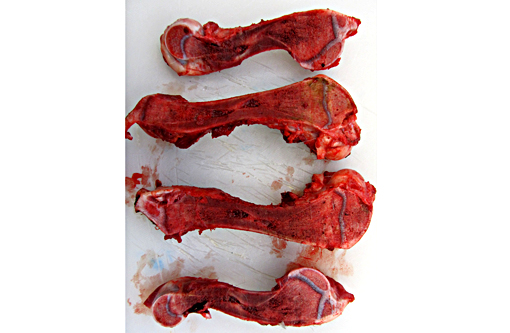
Gross Description:
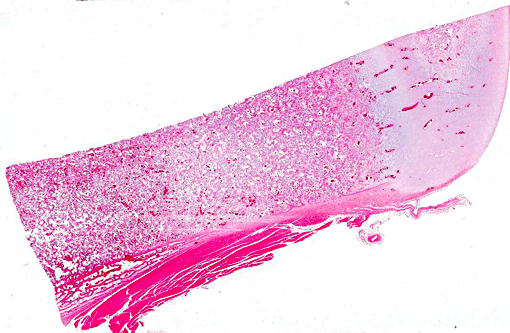
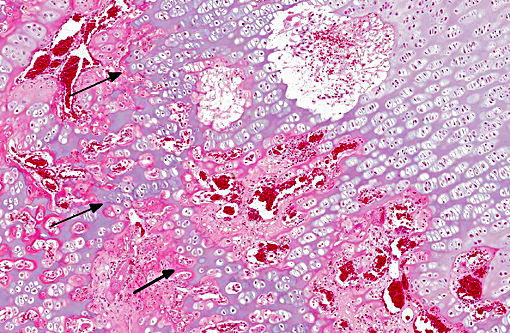
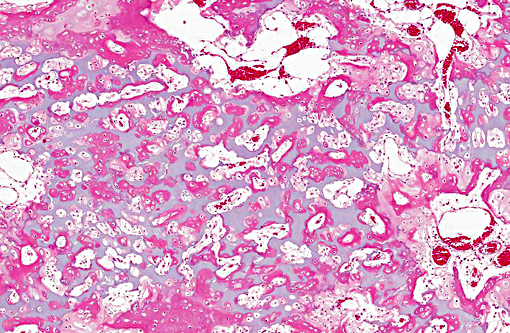
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Osteopetrosis caused by failure of osteoclast development is rare, but osteoclast-poor disease with autosomal recessive heritability exists in humans. Osteoclast differentiation is dependent on RANK/RANKL (Receptor Activator of Nuclear Factor-KB/ Receptor Activator of Nuclear Factor-KB Ligand) signaling, and mutations in genes responsible for this receptor/ligand pair ( TNFRSF11A and TNFRSF11, respectively) lead to the profound osteoclast deficiency.(11,5) In mouse models, osteopetrosis is also observed with defects in a range of other genes associated with osteoclast differentiation, but these have not been described in natural disease.(3) The depletion of osteoclasts in the present case suggests it may be a different form of osteopetrosis to that described in most other equine cases, which typically display normal to increased osteoclast numbers. However, a single case of osteoclast-poor osteopetrosis has been reported in a Peruvian Paso foal.(8)
Acquired osteoporosis like disease has been reported secondary to a number of viral infections, presumably reflecting viral osteoclast tropism and cell depletion. Zonal lesions consistent with osteopetrosis have been reported with bovine viral diarrhoea virus in cattle,(9) and similar features have been identified in the metaphyseal region of dogs with distemper and cats infected with feline leukaemia virus.(12) Osteosclerotic disease similar to osteopetrosis has also been reported associated with hypervitaminosis,(6) lead poisoning,(12) and exogenous oestrogen(5) and glucocorticoid administration.(4) Cases of rickets may display retention of cartilaginous metaphyseal trabeculae overlayed by osteoid, similar to those observed in osteopetrosis. In rickets, however, the trabeculae are poorly mineralized, while the bone in cases of osteopetrosis undergoes normal mineralization.
JPC Diagnosis:
Conference Comment:
There are two main forms of osteopetrosis described in humans: a recessively inherited lethal form in which lesions are present at birth, and a dominant form which manifests in adults.(2) Osteopetrosis in Red Angus calves with a deletion mutation in the gene SLC4A2, which encodes the anion exchanger for carbonate and chloride, has recently been compared to the recessive form which occurs in children, and aspects of the craniofacial lesions were found to be similar. Craniofacial lesions in affected calves include dorsoventrally compressed brains with depressions of the parietal cortex due to thickening of the parietal bone; compression of the cerebral hemispheres with vermis herniation through the foramen magnum; chromatolysis in multiple cranial nerve nuclei; optic nerve atrophy; loss of retinal ganglion cells; and dysplastic changes in the molar and premolar teeth. There was also corpora amylacea in the thalamus, basal nuclei and midbrain, and mineralization in vessels of the thalamus. One important difference noted between the condition in Red Angus calves and the recessive lethal form in humans is the presence of increased numbers of osteoclasts in people, while decreased numbers of osteoclasts were present in the long bones of affected calves. Osteoclast numbers in calves, however, were more normal in bones of the head. Affected calves died in utero or shortly after birth and were either homozygous or heterozygous for the SLC4A2 mutation, respectively.(9) Long bone lesions in affected calves included dense unresorbed bony trabeculae from the metaphysis to the central diaphysis, similar to what is present in other species.(2)
The specific type of osteoclast defect present influences the number of osteoclasts, whether increased, decreased, or absent, in various types of osteopetrosis. For example, in mutations involving the chloride channel (ClCN7) or the proton pump (ATP6i), osteoclasts may be present in increased numbers but are nonfunctional; however, in mutations involving the RANKL gene, necessary for proper osteoclast differentiation, osteoclasts are decreased or absent. Osteopetrosis in Hereford and Simmental breeds of cattle is similar to the condition in Angus calves. One difference is the presence of thickened frontal bones with cystic spaces, creating a domed forehead that can be mistaken for hydrocephalus. Osteopetrosis is described in Belgian Blue cattle in Europe in combination with abnormal skull formation and mandibular gingival hamartomas. A mutation was identified in the chloride / proton exchanger lysosomal anion transporter ClCN7 in affected calves. Osteopetrosis is also described in inbred Polypay sheep and white-tailed deer, both of which also have brachygnathia inferior, a common finding in many affected species. The white-tailed deer also have calluses on several ribs, similar to the disease in horses, suggesting in utero rib fractures. Osteopetrosis has been documented in dogs but is poorly characterized.(2)
References:
1. Berry CR, House JK, Poulos PP, et al. Radiographic and pathologic features of osteopetrosis in two Peruvian Paso foals. Veterinary Radiology & Ultrasound. 1994;35:355-361.
2. Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol1. St. Louis, MO: Elsevier; 2015:49-53.
3. Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19-29.
4. Glade MJ, Krock L. Glucocorticoid-lnduced inhibition of osteolysis and the development of osteopetrosis, osteonecrosis and osteoporosis. Cornell Vet. 1982;72:76-91.
5. Guerrini MM, Sobacchi C, Cassani B, et al. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet. 2008;83:64-76.
6. Kramers P, Fluckiger MA, Rahn BA, et al. Osteopetrosis in Cats. Journal of Small Animal Practice. 1988;29:153-164.
7. Meyers SN, McDaneld TG, Swist SL, et al. A deletion mutation in bovine SLC4A2 is associated with osteopetrosis in Red Angus cattle. BMC Genomics. 2010;11:337.
8. Nation PN, Klavano GG. Osteopetrosis in two foals. Can Vet J. 1986;27:74-7.
9. OToole D, Swist S, Steadman L, Johnson GC. Neuropathology and craniofacial lesions of osteopetrotic Red Angus calves. Vet Pathol. 2012;49(5):746-754.
10. Scruggs OW, Fleming SA, Maslin WR, et al. Osteopetrosis, anemia, thrombocytopenia, and marrow necrosis in beef calves naturally infected with bovine virus diarrhea virus. J Vet Diagn Invest. 1995;7:555-9.
11. Sobacchi C, Frattini A, Guerrini MM, et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet. 2007;39:960-2.
12. Thompson K. Diseases of Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy and Palmer 's Pathology of Domestic Animals. 5th ed. Vol1. Philadelphia, PA: Elsevier Ltd; 2007:24-40, 75-80.
13. Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839-49.