CASE II: 14W4636 (JPC 4068606-00)
Signalment:
Juvenile, male, Rocky mountain elk (Cervus canadensis nelsoni).
History:
Forty-four free-ranging elk calves died on a Wyoming Game and Fish Department winter feedground over the previous month (March). The winter was atypical, even for Wyoming: unusually heavy amounts of wet snow, followed by bouts of above freezing temperatures. Biologists described calves as losing condition and becoming recumbent. This calf was euthanized by gunshot and submitted for necropsy.
Gross Pathology:
The carcass was that of a juvenile male elk in poor nutritional condition. Dead weight: 110 kg. There was serous atrophy of fat throughout. Femoral bone marrow was red and gelatinous. Approximately 25% of the liver had sharply-demarcated caseous necrosis (from pathologist's notes). Interdigital lesions were recorded as present but 'minimal'.
Laboratory Results:
· PCR on liver Fusobacterium necrophorum: detected
· Clostridium perfringens and Trueperella pyogenes isolated (liver)
· Negative on culture for Brucella abortus (multiple lymph nodes)
· Negative IHC for chronic wasting disease (obex; palatine tonsil; medial retropharyngeal LN)
Microscopic Description:
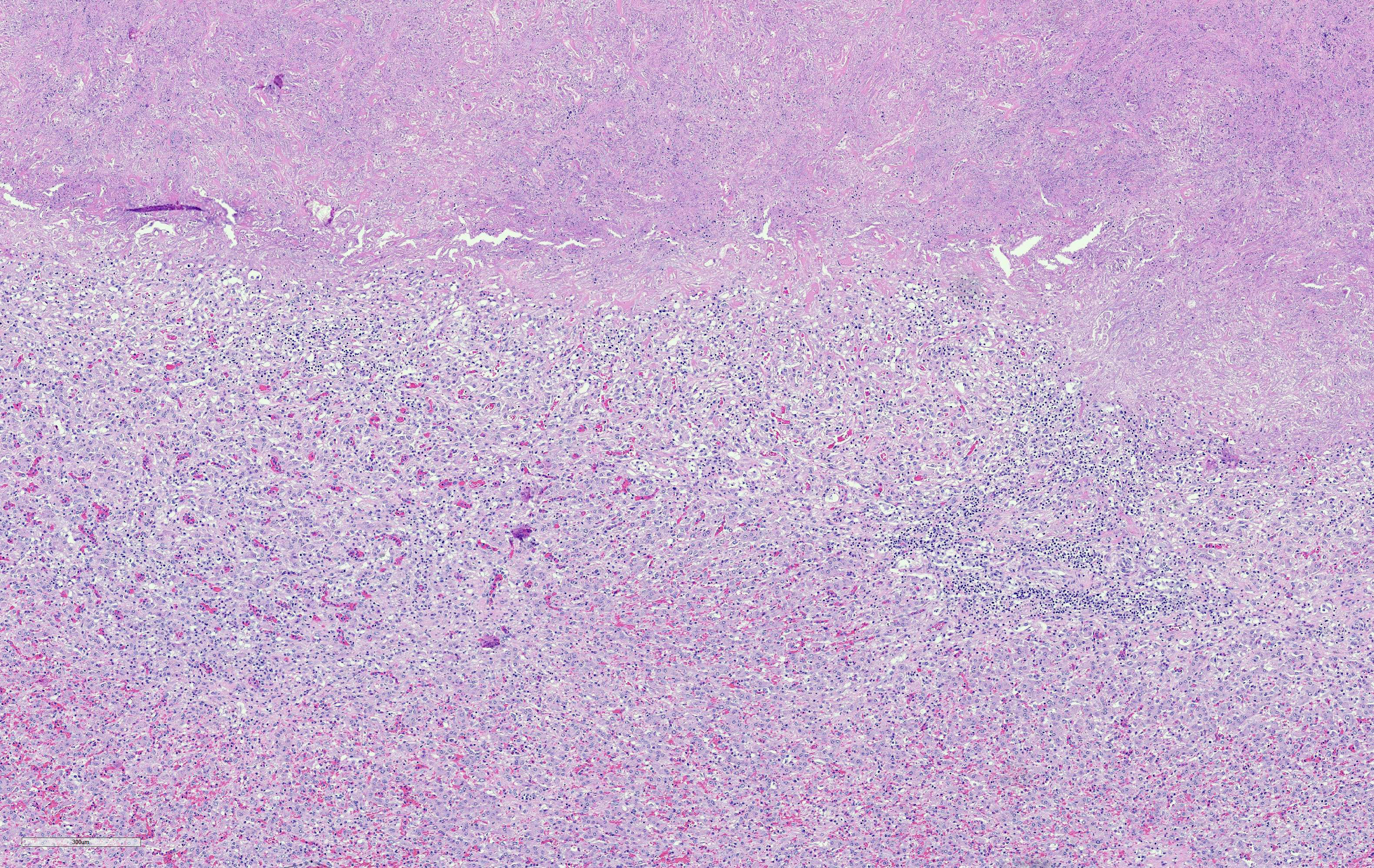
Two tissues are presented. The femoral bone marrow exhibits marked serous atrophy of fat, with atrophic adipocytes separated by expanded eosinophilic intercellular matrix. The section of liver encompasses an abrupt margin between necrotic and viable hepatic tissue. There is fibrosis, disorganization of hepatic plates, and lymphocytic-histiocytic inflammation with neutrophils throughout. Bacteria are present in necrotic tissue close to viable tissue margins. A Gram's stain demonstrates that bacteria are filamentous and Gram-negative. No Clostridium sp. bacteria are seen in special stains.
Contributor's
Morphologic Diagnosis:
1. Serous atrophy
of fat, severe, diffuse.
2. Necrotizing hepatitis, severe, subacute, locally extensive, with intralesional filamentous Gram-negative bacteria.
Contributor's Comment:
Fusobacterium necrophorum is a common opportunistic pathogen familiar to diagnostic pathologists from its involvement in footrot in cattle, interdigital dermatitis in sheep, calf diphtheria, necrotic rhinitis of pigs, and hepatic necrosis in feedlot cattle fed a 'hot' carbohydrate-rich ration. Necrobacillosis is recurrent among free-ranging elk on Wyoming's winter feedgrounds.1 Typically it affects juveniles, which develop a combination of digital and internal lesions. Digital skin and the mouth are presumed portals of entry. The association with crowding elk on winter feedgrounds (in Jackson Hole, WY) was made in the late 1920s by pioneering elk biologist Olaus Murie.2 The cumulative death toll on the feedground in late winter 2014 was 80 elk, but in some years it can be in the several 100s. Other ungulates, when stressed and crowded, are similarly susceptible. They include white-tailed deer (Odocoileus virginianus), mule deer (Odocoileus hemionus), pronghorn (Antilocapra americana), wild and semi-domesticated tundra reindeer (Rangifer tarandus tarandus), and wisent (Bison bonasus);3,4 Wobeser et al's paper has excellent illustrations of the lesions.4 A memorably catastrophic episode involved pronghorn trapped in Wyoming and, for their sins, sent to Texas.5
The overall pattern of losses in elk was typical of necrobacillosis. Elk calves became sick in part due to the wet weather. F. necrophorum is a normal inhabitant of the gastrointestinal system and is present in feces. It thrives in wet anaerobic environments. Multiple freeze/thaw cycles resulted in crusting of snow with sharp, jagged ice and mud. Such conditions predispose to digital dermatitis in elk. Extension to liver is a common sequel, resulting in death. Calves and yearlings are typically most susceptible, but adults can be affected.
Trueperella pyogenes was also cultured from liver. This is a common concurrent synergistic infection in necrobacillosis. T. pyogenes supplies a heat-labile factor which stimulates replication of F. necrophorum. Returning the favor, F. necrophorum produces a leukotoxin which aids survival of T. pyogenes.
There is no vaccine for the disease in wildlife, at least in the United States, partly due to the modest immunological response. Control is by avoiding concentration of animals in environments heavily contaminated with feces; preventing cutaneous or oral lacerations; reducing stress; and avoiding feeding practices that promote oral lesions or ruminal acidosis.
Contributing Institution:
Wyoming State Veterinary Laboratory ? http://www.uwyo.edu/wyovet/
JPC Diagnosis:
1. Liver: Hepatitis, necrotizing, focally extensive, severe, with filamentous bacteria.
2. Bone marrow: Serous atrophy of fat, diffuse, severe with filamentous bacteria.
JPC Comment:
The contributor provides an excellent review of Fusobacterium necrophorum's pathogenesis in elk and in addition to other domestic and wildlife species.
F. necrophorum is an aerotolerant-to-anaerobic, non-motile, non-spore forming, pleomorphic, Gram-negative bacterium that tends to form filaments. The organism is a normal inhabitant of the mammalian gastrointestinal tract, particularly within the rumen, and fecal contamination contributes to the pathogen's distribution and concentration in the environment.1,6 Unable to penetrate healthy epithelium, the organism requires a breach in the epidermal barrier in addition to an anaerobic environment to establish infection. Trauma, ischemia, parasitic and viral infections are common predisposing factors.1
Multiple virulence factors contribute to the pathogenicity of F. necrophorum, including a potent leukotoxin, high levels of endotoxin, hemagglutinin, and a collagenolytic cell wall component responsible for dermotoxic activity. Once established in tissue, F. necrophorum proliferates and causes extensive coagulative necrosis linked to vasculitis, thrombosis, and ischemia, likely induced by these potent toxins.1,6 As mentioned by the contributor, concurrent bacterial infections are facilitated by F. necrophorum's leukotoxin, which induces apoptosis of ruminant leukocytes. Pathogens such as Truperella pyogenes and Staphylococcus aureus lower the oxygen tension and redox potential of the tissue, resulting in a synergistic infection.1
Hepatic necrobacillosis is commonly reported in cattle as the result of ruminal acidosis followed by primary infection of F. necrophorum within the rumen wall and subsequent portal spread. In domestic ruminants, digital necrobacillosis does not commonly result in a septicemia and pedal lesions and are often restricted to one foot.7
One study following an outbreak of digital necrobacillosis in a herd of wild Norwegian reindeer found most lesions were localized to a single foot, similar to as described in cattle.6 Significant pain is associated with this disease and lesions are often severe and chronic. Affected animals are therefore more likely to suffer from poor nutrition, as seen in this case (serous atrophy of fat), and/or predation.6 However, another study of 40 wild-caught pronghorns with either primary pododermatitis or necrotic stomatitis caused by F. necrophorum progressed to produce fatal septicemia with metastatic lesions found in the forestomachs, lung, liver, and cecum in 38 animals.7
During the 1800s and early 1900s, digital necrobacillosis was the most serious problem seen in Norwegian reindeer and was linked to intensive herding practices. High numbers of animals were regularly restricted to small, muddy, and feces-contaminated areas for milking and other activities that ultimately resulted in favorable conditions for transmission. The disease has more or less disappeared from reindeer herds since more extensive hearing practices have been adopted, although outbreaks continue to be reported wild Norwegian reindeer.6
References:
1. Carvallo FR, Uzal FA, Flores C, et al. Alimentary necrobacillosis in alpacas. J Vet Diagn Invest. 2020;32(2):339-343.
2. Kreeger TJ, Cornish T, Creekmore TE, Edwards WH, Tate C. Foot rot. In: Field Guide to Diseases of Wyoming Wildlife, pp. 117-119. Wyoming Game and Fish Department. Cheyenne, WY: 2011.
3. Murie, OJ. An epizootic disease of elk. J. Mammal. 1930;1:214-222
4. Wobeser G, Runge W, Noble D. Necrobacillosis in deer and pronghorn antelope in Saskatchewan. Can Vet J. 1975;16(1):3-9.
5. Handeland K. Fusobacterium necrophorum infection. Gavier-Widén D, Duff JP, Meredith A, eds. onIn: Infectious Diseases of Wild Mammals and Birds in Europe. Wiley-Blackwell, Chichester, West Sussex; 2012:428-430.
6. Handeland K, Boye M, Bergsjø B, Bondal H, Isaksen K, Agerholm JS. Digital necrobacillosis in Norwegian wild tundra reindeer (Rangifer tarandus tarandus). J Comp Pathol. 2010;143(1):29-38.
7. Edwards JF, Davis DS, Roffe TJ, Ramiro-Ibañez F, Elzer PH: 2001, Fusobacteriosis in captive wild-caught pronghorns (Antilocapra americana). Vet Pathol. 38(5):549-552.