Signalment:
Gross Description:
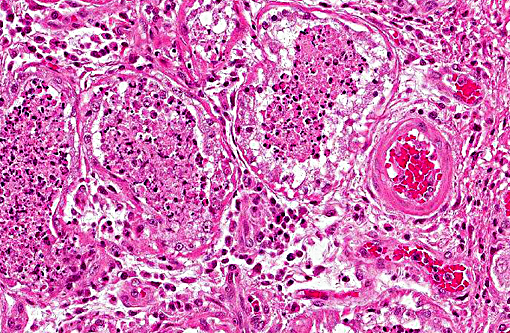
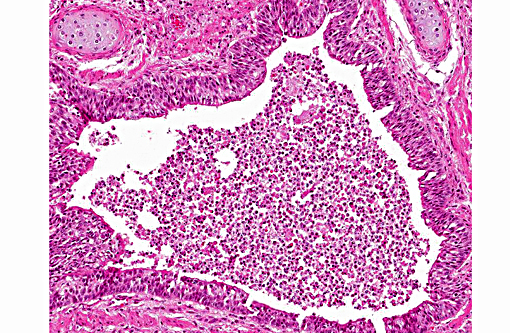
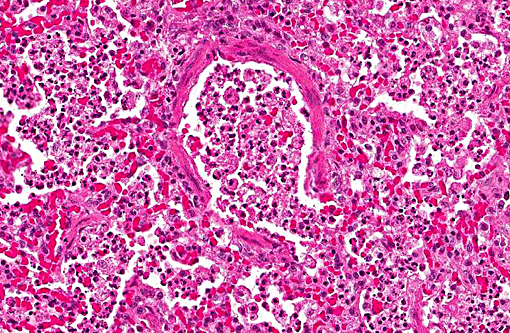
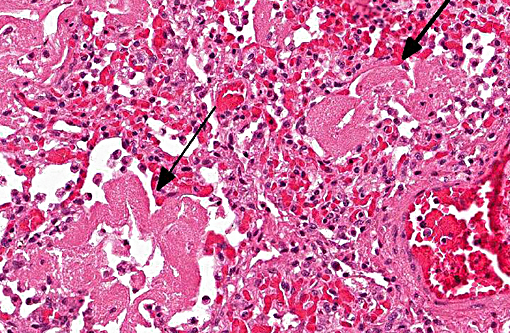
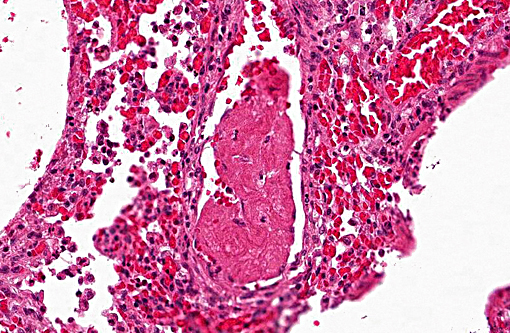
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
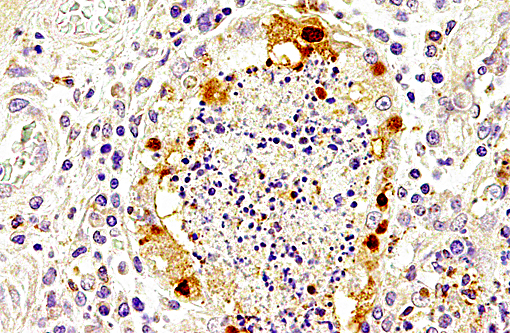
Immunolabeling of influenza A virus nucleoprotein revealed specific antigen staining in the respiratory tract.
Immunohistochemistry for morbillivirus nucleoprotein and reverse transcription PCR for morbillivirus were negative.
Microbiologically, moderate colony numbers of Escherichia coli and low numbers of Acinetobacter pittii, Streptococcus phocae and Stenotrophomonas maltophila were isolated from the lung.
Parasitologically, the lung worms Otostrongylus circumlitus and Parafilaroides gymnurus, as well as the heart worm Acanthocheilonema (previously termed Dipetalonema) spirocauda, were identified in the lungs.
Condition:
Contributor Comment:
Avian influenza A viruses are known to cross species barriers and infect various mammalian species, including man and pinnipeds.(2,4,9,10,13) Different influenza virus A subtypes have previously been reported to cause fatal mass mortalities of harbor seals, including subtypes H5N1, H3N8 and H5N4.(2,4,9,13) However, subtype H10N7 has not been isolated from seals before. Infections with the subtype H10N7 have been reported in man in Egypt and Australia,(3,7) and various avian species including chicken,(3,14) ducks,(14) mallards,(17) and wild birds.(16) According to the predicted amino acid sequence, this subtype is regarded as a low pathogenic avian influenza (LPAI) strain with a low zoonotic potential.(11,17) However, virus detection has been reported in spleen samples of affected seals from Denmark and a more virulent potential of this H10N7 subtype cannot be excluded completely.(11)
The influenza epidemic in 2014 in northern European waters started with an increased mortality among harbor seals in March in Sweden, and swapped in July to Denmark,(11) and reached Germany in October and The Netherlands in November. More than 2400 dead harbor seals were counted in German coastal waters.(4,12) The origin and mode of transmission of this avian influenza virus remains undetermined. Phylogenetic analysis revealed a close relationship to various influenza A viruses detected in wild birds. Specifically, the hemagglutinin and neuraminidase genes were genetically most closely related to subtype H10N7 viruses recently found in migratory ducks in Georgia, Egypt, and the Netherlands.(4) The seals may be infected oronasally with the virus through direct or indirect contact with wild birds or their droppings, because they share the same shoreline habitats as waterfowl.(11,18) Hemagglutinin is the attachment protein of influenza viruses, binding to sialylated glycans on the host cell surface. Avian and swine influenza viruses bind to α2,3 linked sialic acid. In contrast, human and several mammalian influenza A viruses bind to sialylated glycans with an α2,6 linkage to galactose.(1,2)
Virus-associated destruction of epithelial cells in the respiratory tract probably caused a predisposition for secondary bacterial infection resulting in suppurative pneumonia. Together with the parasitic burden in the lung, respiratory insufficiency is regarded as a main cause of death. Bacteriologically, Escherichia coli, Acinetobacter pittii, Streptococcus phocae and Stenotrophomonas maltophila were isolated in the present case. Acinetobacter pittii and Streptococcus phocae are regarded as opportunistic pathogens. Similarly, Streptococcus phocae has been isolated from seals affected with phocine distemper, but has also been associated with starvation and abortion in Cape fur seals (Arctocephalus pusillus pusillus).(7) Interestingly, bacteriological culture of lung samples from other seal carcasses with influenza A H10N7 infection resulted in isolation of bacteria, e.g. Bordetella bronchiseptica and Streptococcus equi ssp. zooepidemicus that were not isolated from lung samples of seal carcasses investigated during the annual health monitoring program. However, these bacterial species were exclusively isolated during the seal die-offs in 2002 and 2014 as a secondary pathogen (Siebert, pers. communication).
Commonly reported nematodes in the lung of harbor seals are Parafilaroides gymnurus, Otostrongylus circumlitus, and the heartworm Acanthocheilonema (previously termed Dipetalonema) spirocauda.(15) Nematodic infections of the respiratory tract of harbor seals represent a common finding, particularly in juvenile individuals.(15) The epithelial hyperplasia of deep airways, as well as the vascular thrombosis, may be related to the parasitic infestation in this case.
As differential diagnosis for infectious seal mass die-offs, phocine distemper virus (PDV) infection has to be considered. Phocine distemper was excluded in this case by reverse transcription PCR (RT-PCR) and immunohistochemistry. The total Wadden Sea seal population has reached a so far unprecedented population size of more than 39,000 animals. A serological survey revealed a lack of antibodies against phocine distemper indicating a high susceptibility for phocine distemper.(6) The current prevalence of influenza virus antibodies in the German harbor seal population is unknown.
JPC Diagnosis:
1. Lung: Pneumonia, bronchointerstitial, necrotizing and fibrinous, diffuse, severe, with submucosal gland necrosis, alveolar and interlobular emphysema, and organizing fibrin thrombi.
2. Lung: Larval nematodes, few (variable across sections).
Conference Comment:
During the outbreak described above, which primarily resulted in seal deaths in Sweden, Denmark and Germany, only low numbers of seals were found dead in the Netherlands; the precise reason for variable mortality is unclear. A study measuring antibody levels of H10N7 (which in some references is termed seal influenza A) in captured or rehabilitated seals in the Netherlands found detectable antibodies in 41% of pups, 10% of weaners and 58% of adults or subadults, indicating infection with this virus may be widespread. Antibody titers were also found in adult grey seals. In subadult harbor and grey seals sampled prior to the 2014-2015 outbreak, only a small number had antibody titers against the virus, which may indicate absence of widespread herd immunity at the start of the outbreak.(5)
The conference histologic description was similar to the contributors histologic description above. There was extensive discussion regarding the vascular changes in the section. There are distinctive fibrin thrombi partially occluding vessel lumina, but there are also vessels which appear to have near complete occlusion by fibroblasts and/or macrophages that are surrounded by concentric layers of fibrous tissue. Participants postulated the vascular changes may represent variable chronicity of the lesions, with the older, mature thrombi demonstrating partial to complete recanalization. Multifocally, hyaline membranes line the alveolar surface and are more prominent in the less atelectatic areas of the lung. Alveolar and interlobular septal emphysema were also described. Multifocally there is hyperplasia of bronchial and bronchiolar epithelium, with loss of cilia in many areas. The contributor provides a detailed description and excellent history regarding the events surrounding this case.
References:
1. Air GM. Influenza virus-glycan interactions. Curr Opin Virol. 2014; 7:128133.
2. Anthony SJ, St Leger JA, Pugliares K, et al. Emergence of fatal avian influenza in New England harbor seals. MBio. 2012; 3:e0016612.
3. Arzey GG, Kirkland PD, Arzey KE, et al. Influenza Virus A (H10N7) in Chickens and Poultry Abattoir Workers, Australia. Emerg Infect Dis. 2012; 18:814816.
4. Bodewes R, Bestebroer TM, van der Vries E, et al. Avian Influenza A (H10N7) VirusAssociated Mass Deaths among Harbor Seals. Emerg Infect Dis. 2015; 21:720722.
5. Bodewes R, Garcia AR, Brasseur SM, Sancehz GJ, et al. Seroprevelance of antibodies against seal influenza A (H10N7) virus in harbor seals and gray seals from the Netherlands. PLoS One. 2015; 10(12):e0144899.
6. Bodewes R, Morick D, van de Bildt MW, Osinga N, Rubio Garc+â-¡a A, S+â-ínchez Contreras GJ, Smits SL, Reperant LA, Kuiken T, Osterhaus AD. Prevalence of phocine distemper virus specific antibodies: bracing for the next seal epizootic in northwestern Europe. Emerg Microbes Infect. 2013;2(1):e3.
7. de la Barrera CA, Reyes-Ter+â-ín G. Influenza: Forecast for a Pandemic. Arch Med Res. 2005; 36:628636.
8. Henton MM, Zapke O, Basson PA. Streptococcus phocae infections associated with starvation in Cape fur seals. J S Afr Vet Assoc. 1999; 70:9899.
9. Hinshaw VS, Bean WJ, Webster RG, et al. Are seals frequently infected with avian influenza viruses? J Virol. 1984; 51:863865.
10. Jensen T, van de Bildt M, Dietz HH, et al. Another phocine distemper outbreak in Europe. Science. 2002; 297:209.
11. Krog JS, Hansen MS, Holm E, et al. Influenza A(H10N7) virus in dead harbor seals, Denmark. Emerg Infect Dis. 2015; 21:684687.
12. Pund R, Huesmann J, Neuhaus H, et al. Auswirkungen des Influenza A-Infektionsgeschehens 2014/2015 auf den Seehundbestand in der Nordsee. Natur und Umweltschutz. 2015; 14:1317.
13. Reperant LA, Rimmelzwaan GF, Kuiken T. Avian influenza viruses in mammals. Rev Sci Tech. 2009; 28:137159.
14. Serena Beato M, Terregino C, Cattoli G, et al. Isolation and characterization of an H10N7 avian influenza virus from poultry carcasses smuggled from China into Italy. Avian Pathol. 2006; 35:400403.
15. Siebert U, Wohlsein P, Lehnert K, et al. Pathological Findings in Harbour Seals (Phoca vitulina): 1996-2005. J. Comp. Path. 2007; 137:4758.
16. Siembieda JL, Johnson CK, Cardona C, et al. Influenza A viruses in wild birds of the Pacific flyway, 2005-2008. Vector Borne Zoonotic Dis. 2010; 10:793800.
17. Vittecoq M, Grandhomme V, Champagnon J, et al. High influenza A virus infection rates in Mallards bred for hunting in the Camargue, South of France. PLoS One. 2012; 7(8):e43974.
18. Zohari S, Neimanis A, H+â-ñrk+â-¦nen T, et al. Avian influenza A (H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Euro Surveill. 2014; 19(46).
19. (http://www.waddenseasecretariat.org/sites/default/files/downloads/TMAP_downloads/Seals/harbour_seal_report_2014_b.pdf).