Signalment:
Gross Description:
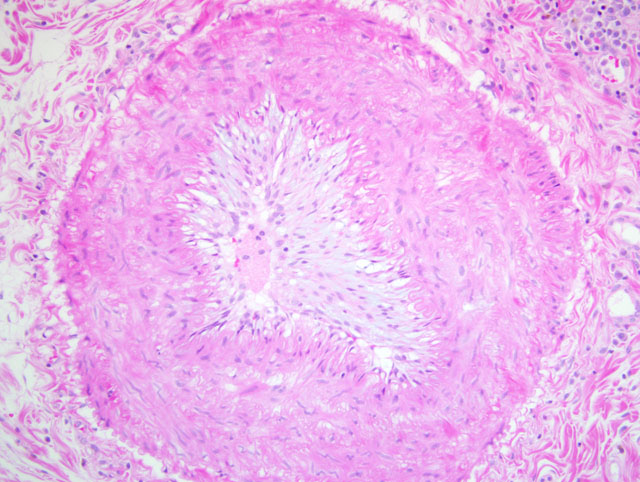
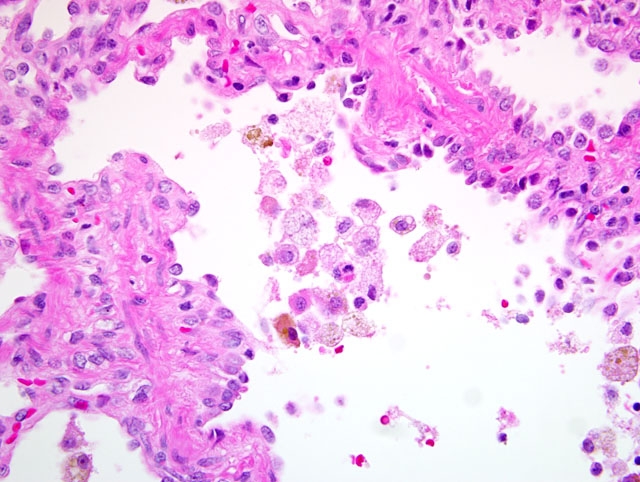
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
| Test and Value | Reference Range | |
| RBC | 5.26 x 106/mm3 | 5.0 - 6.5 x 106/mm3 |
| WBC | 2.0 x 103/mm3 | 6.0 - 15.0 x 103/mm3 |
| Lymphocytes | 56.1% | 25.0 - 60.0% |
| Monocytes | 20.5% | 0.0 - 8.0% |
| Eosinophils | 0.5% | 0.0 - 5.0% |
| Platelets | 66 x 103/mm3 | 330 - 650 x 103/mm3 |
Condition:
Contributor Comment:
Thrombocytopenia usually results from either impaired production or enhanced destruction of platelets. In most cases it is due to immune mediated destruction of platelets or megakaryocytes or increased utilization of platelets as in disseminated intravascular coagulation. Thrombocytopenia is a common complication in HIV patients and SIVinfected macaques.(7) Although several factors attributable to thrombocytopenia have been identified, the precise mechanism of thrombocytopenia is unclear. Autoantibodies against platelet GPIIIa protein induces a hypercoagulable state by interacting with phospholipids and other clotting factors, thus causing platelet aggregation in HIV patients.(7) In SIV-infected rhesus macaques, synthesis of platelet autoantibodies results in increased phagocytosis of platelets.(1) Also, megakaryocytes express CD4 molecules and are productively infected by HIV/ SIV resulting in decreased production of platelets. In addition, SIV-infected macaques develop cold agglutinins resulting in thrombocytopenia.(4)
In this animal, the endothelial cell activation, intimal proliferation, altered hemodynamics and blood hypercoagulability predisposed to clot formation. Vascular obstruction resulted in pulmonary infarction. The cause of thrombocytopenia in this case is most likely multifactorial and may be attributed to destruction of megakaryocytes by replicating virus, production of autoantibodies against platelet autoantigens and/or increased aggregation and removal of platelets.
Infectious diseases associated with thrombocytopenia in animals are provided in the table below:(2,9)
| Species | Etiology |
| Cat | Feline immunodeficiency virus Feline leukemia virus Feline panleukopenia virus |
| Dog | Canine distemper virus Ehrlichia canis |
| Horse | Equine infectious anemia virus Equine arteritis virus African horse sickness virus |
| Cattle | Bovine viral diarrhea virus Theileria parva Trypanosoma congolense Trypanosoma vivax |
| Pig | African swine fever virus Hog cholera virus |
JPC Diagnosis:
1. Lung: Arteriopathy, proliferative, intimal and medial, multifocal, marked, with luminal occulusion, multifocal hemorrhagic coagulative necrosis with peripheral fibrosis (infarcts), and pleural fibrosis.
2. Lung: Alveolar histiocytosis and hemosiderosis, diffuse, mild to moderate, with type II pneumocyte hypertrophy and hyperplasia.
Conference Comment:
Endothelial injury resulting in denudation of the endothelium exposes the subendothelial extracellular matrix, allowing platelet adhesion, release of tissue factor, and local depletion of antithrombotic prostaglandin (PG)I2 and plasminogen activators. More inclusively, this vertex of Virchows triad might be termed endothelial dysfunction, since any disturbance in the balance of pro- and antithrombotic activities may lead to thrombosis. Specifically, dysfunctional endothelium is characterized by relatively increased production of procoagulant factors (e.g. tissue factor, platelet adhesion molecules, plasminogen activator inhibitors) and decreased production of anticoagulant factors (e.g. thrombomodulin, PGI2, tissue plasminogen activator); insults that lead to this type of endothelial dysfunction are varied and include, among others, turbulent blood flow, hypertension, endotoxins, and hypercholesterolemia.(5)
Altered blood flow, or turbulence, not only causes endothelial dysfunction as described above, but also causes the formation of pockets of stasis. Normal blood flow is laminar, which maintains platelets in the center of the vessel lumen; areas of stasis lack laminar flow, bringing platelets into contact with the endothelium and thus promoting thrombosis. Moreover, blood in areas of stasis becomes stagnant; activated clotting factors are not readily diluted and washed away from these areas, and clotting factor inhibitors do not flow into them.(5)
Hypercoagulability, also referred to as thrombophilia, is the third vertex of Virchows triad. While a number of hereditary causes of hypercoagulability are described in human medicine, acquired hypercoagulability is more commonly encountered in veterinary medicine, and is often multifactorial. For instance, coagulation factor I may be elevated due to inflammation, stress, tissue necrosis, while factors I and VIII may be elevated due to trauma or hyperthyroidism. Conversely, depletion of antibrombin III, a common complication of the nephrotic syndrome, leads to hypercoagulability via loss of thrombin inhibition.(5,6)
The conference moderator noted that anatomic location is of critical importance in determining the consequences of thrombosis. While thrombosis in the heart or brain is likely to cause catastrophic infarction, organs like the liver and lung which have collateral circulation, are less prone to clinically significant infarction.
Selected causes of thrombosis, categorized by their respective vertices of Virchows triad, are summarized below:(6)
| Causes of Thrombosis | |
|---|---|
| Mechanism | Examples |
| Endothelial Injury / Dysfunction | Nematodiasis (Strongylus vulgaris larvae, dirofilariasis, angiostrongylosis, spirocercosis)
Bacterial infection (salmonellosis, mannheimiosis, erysipelas, histophilosis) Viral infection (arterivirus, morbillivirus, herpesvirus, orbivirus, pestivirus) Fungal infection (aspergillosis, zygomycosis) Disseminated intravascular coagulation (DIC) Vitamin E / selenium deficiency Immune-mediated vasculitis Endotoxin |
| Altered Blood Flow | Gastric dilation and volvulus
Intestinal torsion or volvulus Cardiomyopathy Hypovolemia Aneurysm |
| Hypercoagulability | Increased clotting factor activation (neoplasia, DIC)
Antithrombin III deficiency (nephrotic syndrome, DIC, liver disease) Increased platelet activity (diabetes mellitus, neoplasia, dirofilariasis, uremia) Metabolic abnormalities (hyperthyroidism, hyperadrenocorticism) |
References:
2. Jones TC, Hunt RD, King NW: Veterinary Pathology, 6th ed., p. 161. Williams & Wilkins, Baltimore, MD 1997
3. Kuwata T, Nishimura Y, Whitted S, Ourmanov I, Brown CR, Dang Q, Buckler-White A, Iyengar R, Brenchley JM, Hirsch VM: Association of progressive CD4(+) T cell decline in SIV infection with the induction of autoreactive antibodies. PLoS Pathog 5:e1000372. Epub 2009
4. Mandell CP, Spinner A: Cold agglutinins in rhesus macaques infected with simian immunodeficiency virus. Compara Haem Inter 7:238-242, 1997
5. Mitchell RN: Hemodynamic disorders, thromboembolic disease, and shock. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 121-126. Saunders Elsevier, Philadelphia, PA, 2010
6. Mosier DA: Vascular disorders and thrombosis. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 86-91. Mosby Elsevier, St. Louis, MO, 2007
7. Onlamoon N, Pattanapanyasat K, Ansari A: Human and nonhuman lentiviral infection and autoimmunity. Ann NY Acad Sci 1050:397-409, 2005
8. Perez-Atayde AR, Kearney DI, Bricker JT, Colan SD, Easley KA, Kaplan S, Lai WW, Lipshultz SE, Moodie DS, Sopko G, Starc TJ, P2C2 HIV Study Group: Cardiac, aortic, and pulmonary arteriopathy in HIV-infected children: the prospective P2C2 HIV multicenter study. Pediatr Dev Pathol 7:61-70, 2004
9. Russell KE, Grindem CB: Secondary thrombocytopenia. In: Schalm's Veterinary Hematology, eds. Feldman BF, Zinkl JG, Jain NC, 5th ed., pp. 487-495. Lippincott Williams & Wilkins, Baltimore, MD, 2000
10. Shannon RP, Simon MA, Mathier MA, Geng YJ, Mankad S, Lackner AA: Dilated cardiomyopathy associated with simian AIDS in nonhuman primates. Circulation 101:185-193, 2000
11. Yanai T, Lackner AA, Sakai H, Masegi T, Simon MA: Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta). J Med Primatol 35:106-112, 2006