Signalment:
Gross Description:
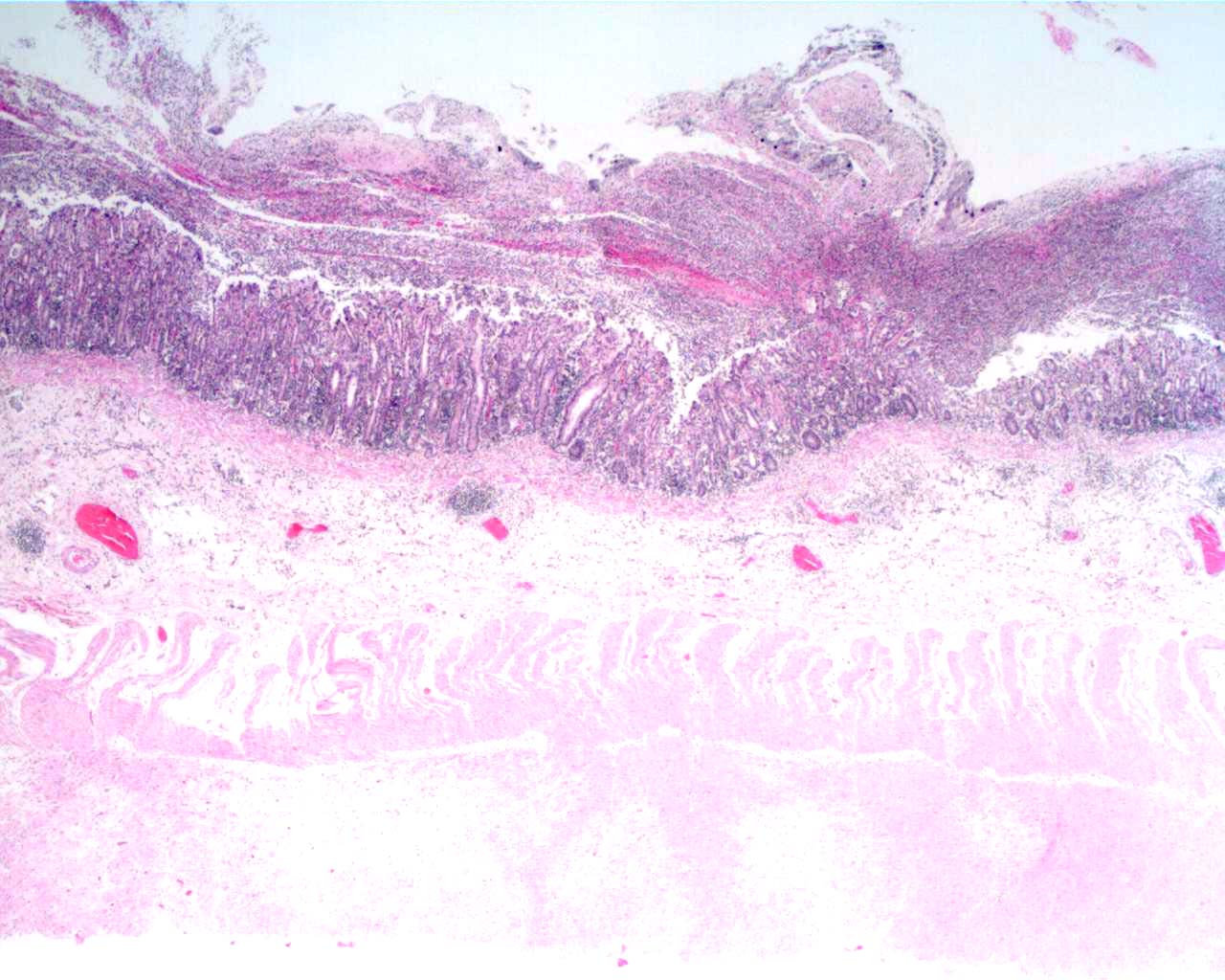
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Shigella flexneri, Yersinia enterocolitica, Strongyloides fulleborni, adenovirus, Campylobacter coli and C. jejuni were recently reported to be associated with chronic diarrhea in Rhesus macaques.7 Enteric agents not associated with chronic diarrhea within the same population included Balantidium coli, Giardia lamblia, Trichuris trichiura, Enterocytozoon bieneusi and enteropathogenic E. coli carrying the eaeA intimin or Stx2c Shiga toxin virulent genes. Chronic diarrhea was associated with increased CD69+ cells and CD4+ T lymphocytes as well as upregulation of IL-1-α, IL3, and TNF-α cytokine genes. Chronic diarrhea is the leading cause of morbidity requiring veterinary care in captive primates and is not currently a criterion of specific pathogen-free colonies. The enteric infections and associated modulation of the immune system is a complicating variable in the study of AIDS and SIV in the primate models.Â
JPC Diagnosis:
Conference Comment:
Infection and disease does not provide immunity. Animals with shigellosis may be chronically reinfected, and colonies endemically infected my not demonstrate overt clinical disease unless challenged by a stressful event or compromised immune system.3
Upon ingestion of the bacteria, it is proposed that invasion of the M cells overlaying lymphoid follicles within the colon, allows the bacteria to reach the basolateral pole of the epithelial cells, which is where they induce their entry.5
Shigellosis in humans is caused by both Shigella spp. and enteroinvasive Escherichia coli.5 These bacteria contain a virulence plasmid that encodes most proteins directly involved in host cell entry, bacterial dissemination, and induction of apoptosis in infected macrophages.4 This region encodes a type III secretion apparatus (Mxi-Spa TTS apparatus), translocators (IpaB and Ipa C), effectors (IpaD, IpgB1, IpgD and IcsB), their dedicated chaperones (IpgA, IpgC, IpgE and Spa15), and two transcriptional activators (VirB and MxiE). The current theory suggests that upon contact of the bacterium with the host cell, translocators are inserted into the host cell membrane which forms a pore, effectors are then transmitted through this pore and enter into the host cell cytoplasm.
Genes encoding a type III secretory apparatus have been identified in a number of mammalian and plant pathogens including enterohemorrhagic and enteropathogenic Escherichia coli, Shigella, Salmonella, Yersinia, Chlamydia, Bordetella, Pseudomonas, Xanthomonas, Ralstonia, and Erwinia spp.6
References:
2. Benirschke K, Garner FM, Jones TC: Pathology of Laboratory Animals, vol. II, pp 1449, 1978
3. Bernacky BJ, Gibson SV, Keeling ME, Abee CR: Nonhuman primates. In: Laboratory Animal Medicine, eds. Fox JG, Anderson LC, Loew FM, Quimby FW, 2nd ed., pp. 730-734. Elsevier Science, San Diego, CA, 2002
4. Gall TL, Mavris M, Martino MC, Bernardini ML, Denamur E, Parsot: Analysis of virulence plasmid gene expression defines three classes of effectors in the type III secretion system of Shigella flexneri. Microbiol 151:951-962, 2005
5. Parsot C: Shigella spp. and enteroinvasive Escherichia coli pathogenicity factors. FEMS Microbiol Let 252:11-18, 2005
6. Schuch R, Maurelli AT: The Mxi-Spa Type III secretory pathway of Shigella flexneri requires an outer membrane lipoprotein, MxiM, for invasion translocation. Infect Immun 67:1982-1991, 1999
7. Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA: Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun 71:4079-4086, 2003