Wednesday Slide Conference, Conference 5, Case 1
Signalment:
Six years-old male common woolly monkey (Lagothrix lagotricha).
History:
The animal was housed at the BH-ZOO (Belo Horizonte, Brazil) and developed acute lethargy, with hypoglycemia, apathy, and hypothermia. Ultrasound examination demonstrated abundant fluid in the abdominal cavity. The animal was treated with therapeutic doses of ceftriaxone, dexamethasone, and morphine, but did not respond to treatment and died two days later.
Gross Pathology:
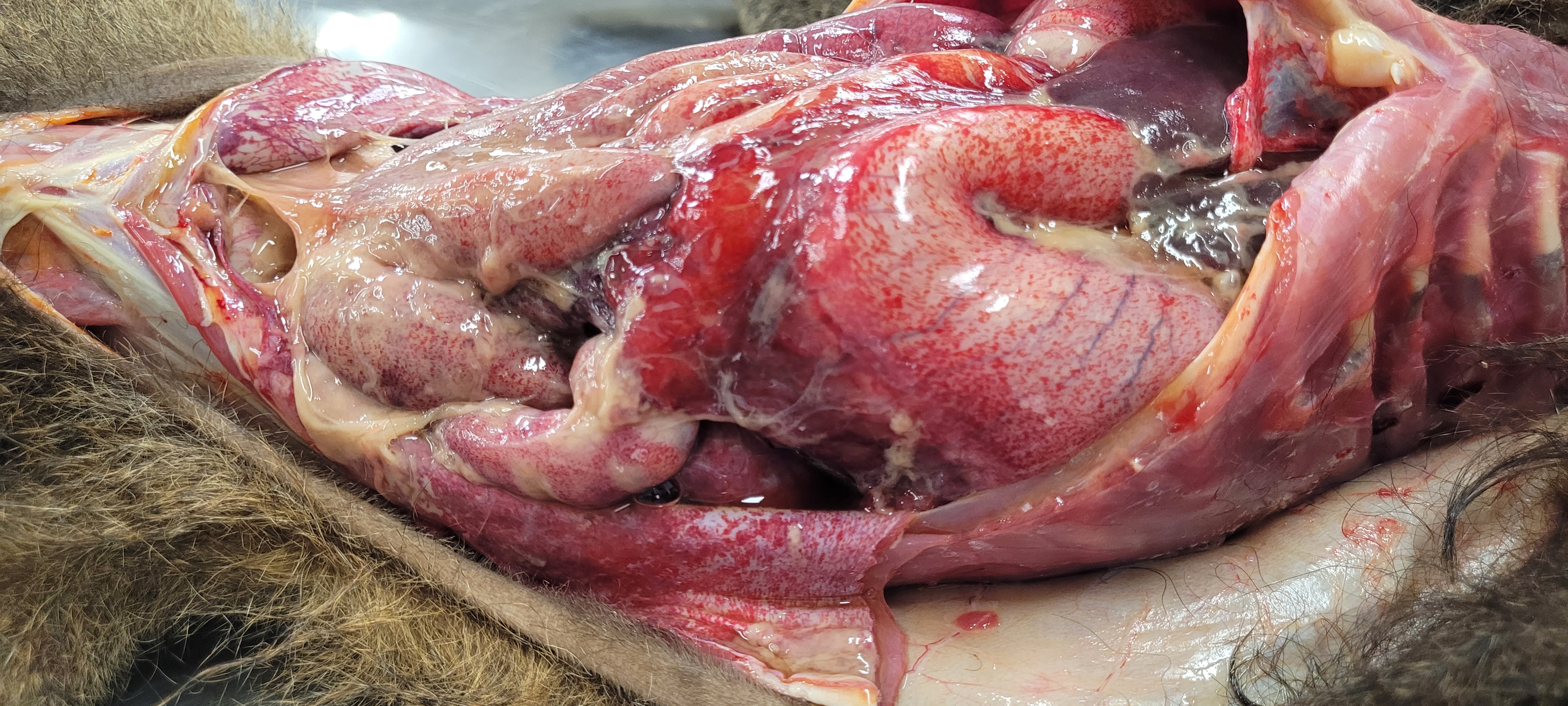
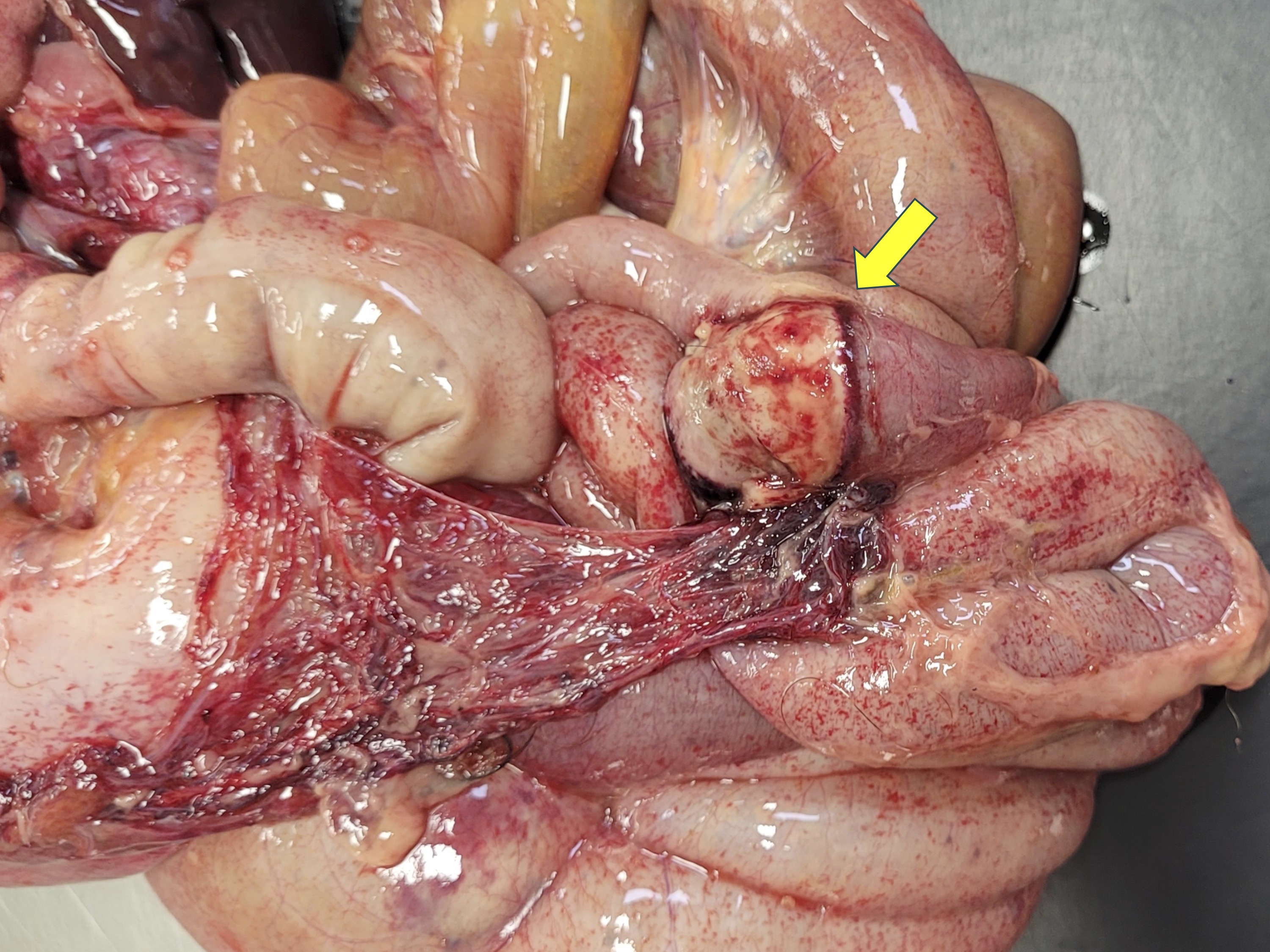
There was abundant yellow fibrinous exudate in the peritoneal cavity, with multifocal to coalescent hemorrhage on the serosa. Gastric and intestinal serosae had multiple red, firms, nodules ranging from 0.1 to 0.2 cm in diameter. The gastric mucosa had multifocal ulcerations with approximately 0.2 cm in diameter. There were multiple areas of fibrinous adhesion between intestinal loops, associated with a necrotic area in the jejunum. The intestinal mucosa had moderate multifocal hemorrhage. There were also multifocal hemorrhages on the mucosa of the urinary bladder. Thoracic cavity and pericardial sac had moderate amounts of translucent fluid with a small amount of fibrin. The lungs had a smooth shiny red surface, with atelectasis in left and right cranial lobes.
Laboratory Results:
Swabs of peritoneal cavity were plated on Muller-Hinton agar (Difco, USA) supplemented with 5% equine blood, mannitol salt agar (Kasvi, Brazil), and MacConkey agar (Kasvi, Brazil), followed by aerobic incubation at 37ºC for 48 h. Colonies were subjected to matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF, Bruker Daltonics) mass spectrometry with results compatible with E. coli. The E. coli isolate was positive for several virulence factors encoding genes: fimH, focG, papC, papG, sfaS, cnf1, usp, hlyA, iutA, and traT. No other bacteria were isolated.
Samples of liver and spleen tested negative for yellow fever virus and dengue virus infections by RT-qPCR.
Microscopic Description:
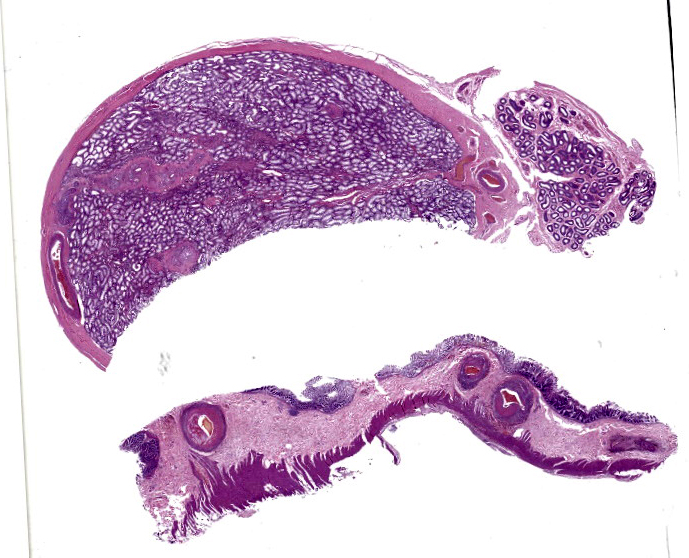
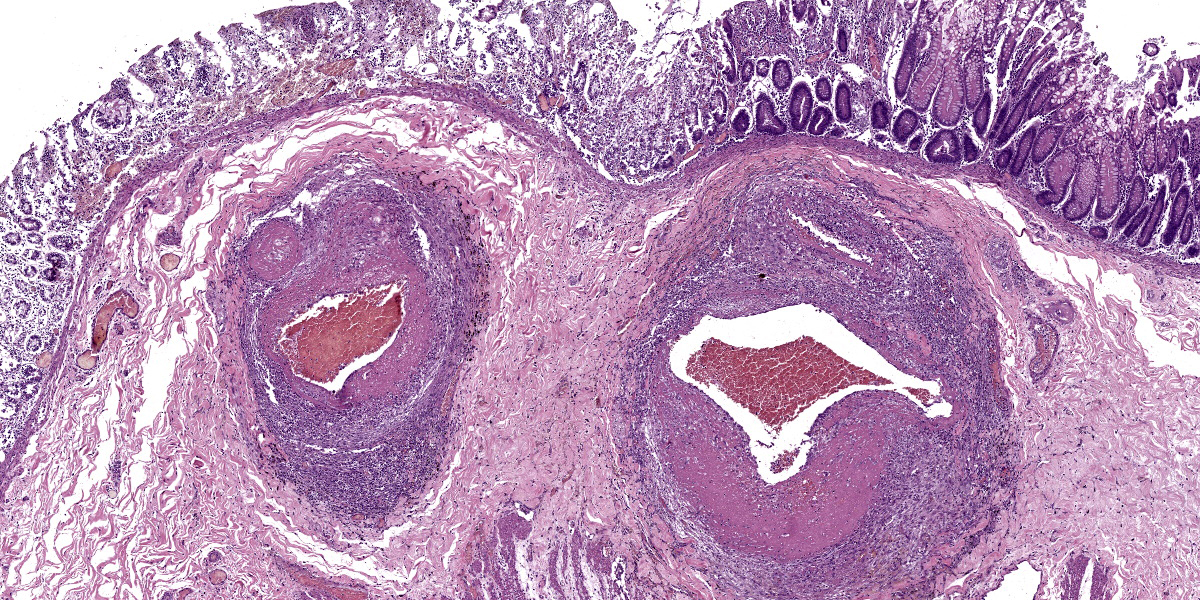
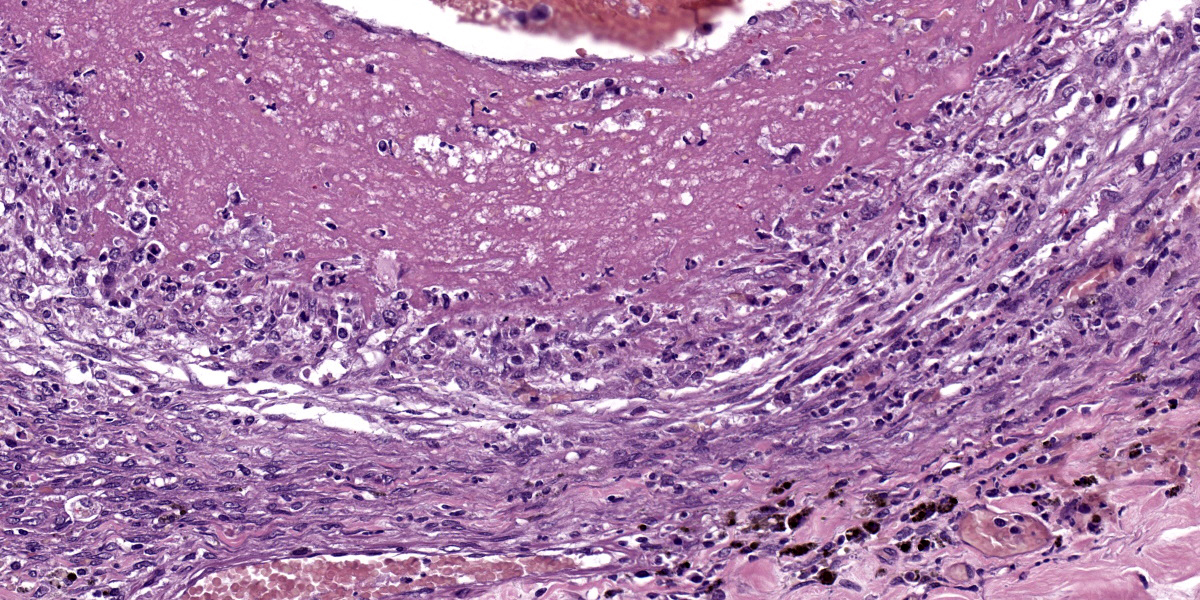
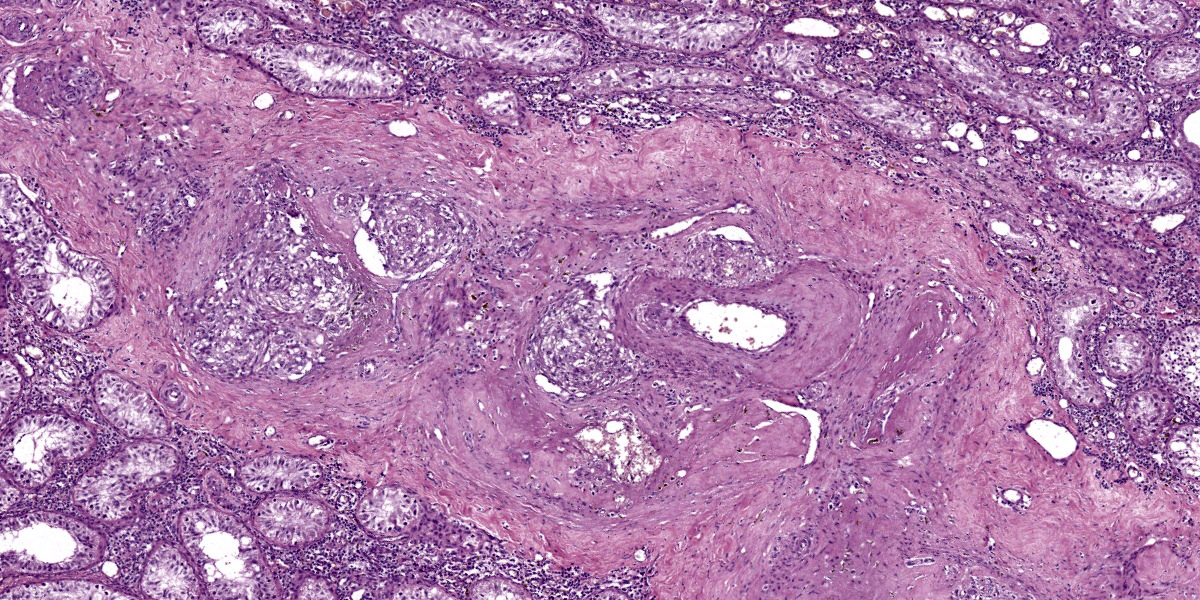
Large intestine: submucosa was expanded with small to medium sized arteries exhibiting variable degrees of inflammation, characterized by infiltration of the tunica adventitia and media with small to moderate numbers of lymphocytes, histiocytes, neutrophils, and some plasma cells. Some of the affected vessels had proliferation of fusiform cells with a small amount of extracellular mucinous matrix within the intima and marked accumulation of collagen in the tunica media and adventitia. Picrosirius red stained sections examined under polarized light demonstrated predominantly reddish birefringent mature type I collagen. Macrophages rich in intracytoplasmic brown pigment (suggestive of hemosiderin) were observed adjacent some of these vessels. In some sections there was marked arterial fibrinoid necrosis affecting the tunica media, often associated with thrombosis. Distribution of arterial lesions was random and segmental. The muscular layer adjacent to the areas of vascular lesion had moderate myocyte hypotrophy. Serosa had a diffuse and moderate inflammatory infiltrate, composed mainly by neutrophils, and the mesothelial cells were diffusely hypertrophic with deposition of moderate amount of fibrin at the serosa associated with neutrophils and histiocytes (peritonitis). Additionally, it was observed a moderate focally extensive area of hemorrhage at the mucosa.
Testis: similar vascular changes were observed in the testis. In addition, seminiferous tubules were marked degenerate with vacuolated Sertoli cells, thickened basal membrane and some sclerotic tubules. Diffusely, there was moderate interstitial infiltrate composed mainly by lymphocytes, histiocytes and plasma cells.
Vascular lesions were observed also at the kidneys, small intestines and stomach. At the small intestine, vascular lesions were associated with multiple focally extensive areas of transmural necrosis. Other lesions included diffuse fibrinous peritonitis, characterized by large amounts of eosinophilic fibrinous exudate with numerous neutrophils, Gram-positive and Gram-negative coccobacilli, and a few vegetal fibers. Mild pulmonary edema was also noted.
Contributor’s Morphologic Diagnoses:
Large intestine: arteritis, necrotizing and proliferative, diffuse, severe with fibrinoid necrosis, thrombosis, and fibrinous peritonitis.
Testis: 1) arteritis, necrotizing and proliferative, diffuse, severe with fibrinoid necrosis and thrombosis. 2) orchitis, lymphoplasmacytic, diffuse, moderate with tubular degeneration, diffuse, moderate.
Contributor’s Comment:
Vascular lesions in this common woolly monkey were consistent with polyarteritis nodosa (PAN) affecting small to medium sized arteries in the kidney, testis, stomach, small, and large intestines. This is the first reported case of PAN in this neotropical primate species. PAN is a progressive, degenerative, inflammatory, and necrotizing disease that most commonly affects small to large arteries of the mesentery, pancreas, kidney, testis, intestine, and heart, as described in humans,6,14 cynomolgus macaques, rhesus monkey, owl monkey, and pigs.2,8,9,16 The diverse types of vascular lesions suggest that the damage is polyphasic, i.e. it occurs intermittently.6 Although the pathogenesis of PAN is not completely clear, it is considered a type III hyper-sensitivity reaction caused by deposition of antigen/antibody complexes in small arteries followed by complement activation.9
PAN is a systemic necrotizing vasculitis, which affects predominantly medium size arteries. It has been described in humans, occurring more often in young adults. Small arteries may also be involved, but there is no involvement of smaller vessels, including arterioles, capillaries, and venules.6 In animals, PAN has been reported in sheep,15 dogs,21 cats,18 pigs,12 and rats.10 Among non-human primates, the disease has been described in cynomolgus monkey (Macaca fasciculres),2,17 rhesus monkey (Macaca mulatta),9 and owl monkey (Aotus sp.).8
In this case, PAN lesions were associated with transmural intestinal necrosis that resulted in peritonitis. PAN often results in narrowing of the vascular lumen due to vasculitis, with secondary gastrointestinal ulcerations or erosions due to ischemia.1 Intestinal perforation or necrosis caused by PAN resulting in acute peritonitis has been reported in humans.11
Pathogenic E. coli was isolated from the peritoneal cavity in this case. E. coli is a major cause of extra-intestinal infections including meningitis, bacteremia, pyelonephritis, cystitis, prostatitis, metritis, and peritonitis.7,20 However, it is also a member of the intestinal microbiota in mammals.19 Extra-intestinal pathogenic E. coli and commensal E. coli typically differ in virulence factors.7,20 In this case, the E. coli isolate was positive for several virulence factors encoding genes: fimH, focG, papC, papG, sfaS, cnf1, usp, hlyA, iutA, and traT.
In conclusion, this is a case of PAN associated with transmural intestinal necrosis and lethal septic peritonitis in a common woolly monkey, which has been recently published.3 Therefore, PAN must be considered for the differential diagnosis of inflammatory, necrotizing and/or proliferative multisystemic segmental arterial lesions in neotropical primates.
Contributing Institution:
Departamento de Clínica e Cirurgia Veterinária, Escola de Veterinária, Universidade Federal de Minas Gerais, Av. Presidente Antônio Carlos, 6627 – CEP 30161-970, Belo Horizonte, MG, Brazil. www.vet.ufmg.br
JPC Diagnoses:
Colon and testis: Arteritis, proliferative, necrotizing, fibronous, chronic, multifocal, severe.
Testis: Orchitis, lymphoplasmacytic, chronic, multifocal, mild.
Colon: Serositis, fibrinous, subacute, diffuse, moderate.
JPC Comment:
This week’s conference was moderated by Major Janas Gray, presently at the US Army Medical Research Institute of Chemical Defense (USAMRICD) in Aberdeen, Maryland. This first case is a terrific example of polyarteritis (periarteritis) nodosa (PAN). Although we have covered PAN in laboratory species before (most recently Conference 1, Case, 2018-2019 in a rat) we have also seen similar lesions in a cat (Conference 12, Case 4, 2022-2023), so this entity should be considered as a differential in any case of systemic necrotizing vasculitis. In particular, the sections of intestine and testis from this monkey feature robust infiltration of the adventitia and outer media by inflammatory cells (Figures 1-3 and 1-4).
This case is unusual in the degree of secondary changes present in which generated lengthy discussion. Conference participants largely agreed with the contributor’s interpretation of the case but differed on how to best interpret the testis. Given that the arteritis was only multifocal, diffuse tubular changes were harder to support. In section, participants noted few spermatids within the epididymis as well as spermatogonia and spermatocytes within the seminiferous tubules, and coupled with the finding that this young individual was entering the phase of sexual maturity reported in this species15 led some participants to conclude that the testis was immature rather than senescent.
Another consideration for vascular lesions in the woolly monkey is idiopathic (essential) hypertension.4,5 Microscopic features of this entity include hyaline arteriosclerosis with intramural deposition of amorphous, brightly eosinophilic material and hyperplastic arteriosclerosis with smooth muscle proliferation of the tunica intima and media. Small muscular arteries and arterioles are classically involved and the kidney is the principal organ, though changes in other arteries have been described. A recent case series looking at similar lesions in pygmy marmosets4 identified a small number of non-renal, non cardio-pulmonary in the inner retina, stomach, spleen, and cerebrum. In the present case, the intact inner elastic lamina and lack of overt hyalinization are against this particular interpretation.
As a final aside for this case, PAN has been reported as a sequalae to human COVID-19 vaccination in line with the type III hypersensitivity reaction and resulting antigen/antibody complex pathogenesis the contributor lays out. Although PAN in humans has been associated previously with hepatitis B vaccination, the production of viral protein through mRNA vaccines may inadvertently drive T-lymphocyte autoreactivity and resulting complement activation and cytokine cascade.13
References:
- Agard C, Mouthon L, Mahr A, et al. Microscopic polyangiitis and polyarteritis nodosa: how and when do they start? Arthritis Rheum. 2003;49:709-715.
- Albassam MA, Lillie LE, Smith GS. Asymptomatic polyarteritis in a cynomolgus monkey. Lab Anim Sci.1993;43:628-629.
- Carvalho TP, Oliveira Santos D, Oliveira AR, et al. Polyarteritis nodosa in a captive common wooly monkey (Lagothrix lagotricha) associated with intestinal necrosis, peritonitis, and sepsis. J Med Primatol. 2023;52:197-200.
- Cooley AJ, Savage A, Snowdon CT. Vascular, cardiac, and renal lesions attributed to primary systemic hypertension in western pygmy marmosets (Cebuella pygmaea). Vet Pathol. 2022; 59(2): 358-370.
- Giddens WE, Combs CA, Smith OA, et al. Spontaneous hypertension and its sequelae in woolly monkeys (Lagothrix lagotricha). Lab Anim Sci. 1987;37(6):750–756.
- Guillevin L. Polyarteritis nodosa and microscopic polyangiitis. In: Ball GV, ed. Vasculitis. Bridges; 2012:34-52.
- Johnson JR, Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181:261-272.
- Joint Pathology Center. Veterinary pathology service. In: Wednesday Slide Conference Proceedings. 2012- 2013; Conf 4, Case III, Accessed http://www.askjpc.org/wsco/wsc_showcase4.php?id=YzZnSjlnRzBkTytDcE5QWm5EbUZCUT09 (Accessed 6 July 2022); 2012.
- Joint Pathology Center. Veterinary pathology service. In: Wednesday Slide Conference Proceedings. 2016-2017; Conf 3, Case III http://www.askjpc.org/wsco/wsc_showcase4.php?id=SXgweHNQbEpUS2JUUmhNcDlHelNYdz09 (Accessed 6 July 2022); 2016.
- Lepherd ML, Schlafer DH, De Matos R, et al. Pathology in pratice. Polyarteritis nodosa. J Am Vet Med Assoc. 2013;243:1399-1401.
- Levine SM, Hellmann DB, Stone JH. Gastrointestinal involvement in polyarteritis nodosa (1986-2000): presentation and outcomes in 24 patients. Am J Med. 2002;112:386-391.
- Liu CH, Chiang YH, Chu RM, et al. High incidence of polyarteritis nodosa in the brains of culled sows. J Vet Med Sci. 2005;67:125-127.
- Makiyama A, Abe Y, Furusawa H, Kogami M, Ando T, Tada K, Onimaru M, Ishizu A, Yamaji K, Tamura N. Polyarteritis nodosa diagnosed in a young male after COVID-19 vaccine: A case report. Mod Rheumatol Case Rep. 2023 Dec 29;8(1):125-132.
- Mitchell RN, Halushka MK. Blood Vessels. Kumar V, Abbas AK, Fausto N, eds. In: Robins and Cotran Pathologic Basis of Disease. Elsevier, 2015;509-510.
- Pereira T. H d. S., Mayor, P., Evangelista, J. S. A. M., Lima, A. K. F., de Andrade, R. d. S., & Monteiro, F. O. B. (2024). Reproductive physiology with emphasis on endometrial cycles of woolly and uakari monkeys—A literature review. American Journal of Primatology, 86, e23585.
- Pesavento PA, Dange RB, Ferreras MC, et al. Systemic necrotizing vasculitis in sheep is associated with ovine herpesvirus 2. Vet Pathol. 2019;56:87-92.
- Porter BF, Frost P, Hubbard GB. Polyarteritis nodosa in cynomolgus macaque (Macaca fascicularis). Vet Pathol. 2003;40:570-573.
- Salvadori C, Vezzosi T, Marchetti V, et al. Polyarteritis nodosa in a cat with involvement of the central and peripheral nervous system. J Comp Pathol. 2019;167:6-11.
- Selander RK, Levin BR. Genetic diversity and structure in Escherichia coli populations. Science. 1980;210:545-547.
- Siqueira AK, Ribeiro MG, Leite DS, et al. Virulence factors in Escherichia coli strains isolated from urinary tract infection and pyometra cases and from feces of healthy dogs. Res Vet Sci. 2009;86:206-210.
- Snyder PW, Kazacos EA, Scott-Moncrieff JC, et al. Pathologic Features of naturally occurring juvenile polyarteritis in beagle dog. Vet Pathol. 1995;32:337-345.