Signalment:
Gross Description:
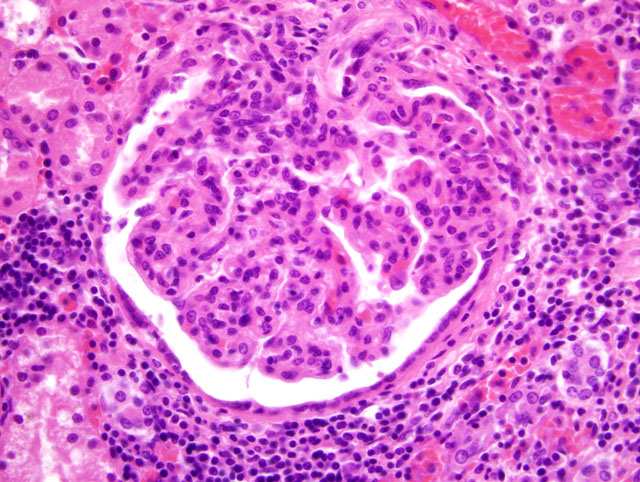
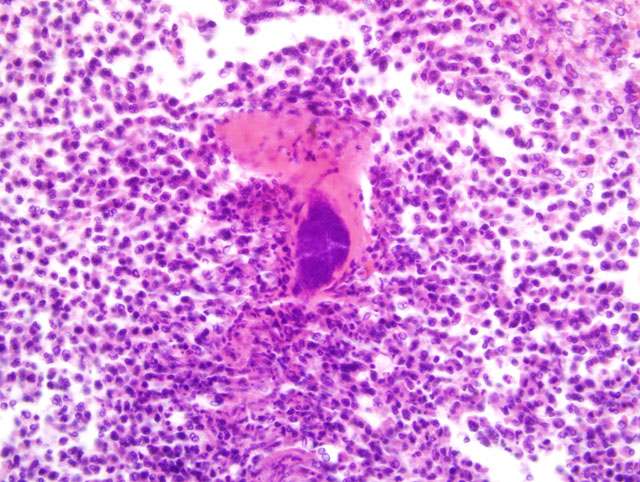
Histopathologic Description:
Morphologic Diagnosis:
1. Infarction and abscessation, multifocal, subacute, severe with coagulative tubular necrosis and bacterial (cocci) colonies.
2. Glomerulopathy, membranoproliferative and mesangioproliferative, diffuse, severe with mild sclerosis.
3. Nephritis, interstitial, lymphoplasmacytic, chronic diffuse, mild.
4. Thrombosis, arcuate artery, focal.
Lab Results:
CBC and clinical chemistry: PCV 30%, WBC 16 k/μl (PMN 90% with 5% bands), BUN 28 mg/dL, creatinine 0.9 mg/dL, ALT 131 U/L, total protein 6.7 g/dL
Microbiology of port area: Staphylococcus spp., coagulase positive, enrofloxacin resistant.
Condition:
Contributor Comment:
Indwelling catheters are convenient tools for the administration of compounds tested for pharmaceutical safety and effectiveness research. Cynomolgus monkeys, rhesus monkeys and baboons are commonly used these studies, often involving psychoactive compounds with subsequent behavioral testing paradigms. Catheters may be tolerated successfully for years if appropriate care is taken to prevent infection. The use of vascular access ports has reduced the numbers of infections due to the closure and healing of the skin, preventing casual exposure. However, ports or extravasated materials into the port pocket may cause focal necrosis with ulceration and exposure of the apparatus.
The formation of infarcts associated with thrombosis and embolization is not as difficult a concept as the pathogenesis of immune complex glomerulonephritis. Several texts have covered this subject extensively, and participants are encouraged to review the mechanisms of membranous, membranoproliferative, and mesangioproliferative glomerular changes.
Several studies of indwelling catheters in baboons have revealed infarcts, septic embolic nephritis and mesangioproliferative glomerulonephritis. Bacteria isolated were Aciteobacter (Herella) sp., Streptococcus sp., Kleibsiella sp., Staphylococcus sp., and Providencia sp. Immunofluorescence studies in six animals on frozen sections revealed granular deposits of IgG (6/6), IgM (5/6), C3 (4/6), and IgA and C4 (2/6) in glomerular lesions. Additionally the IgG deposits correlated with the severity of the lesions. Bacterial antigens were seen in three of six cases, strongly suggesting immunological mediation.(3) Baboons in a second study demonstrated renal and hepatic impairment related to long-term catheterization. The renal lesion in that study was described as membranoproliferative glomerulonephritis with dense deposits noted ultrastructurally in a variety of locations, with mesangial cell interpositioning and foot process fusion. These alterations were found in conjunction with the isolation of Staphylococcus aureus from the blood and catheters.(4) Sheep have been used to study renal infarcts and chronic indwelling catheters. Bacterial species isolated differed from those found in primates and humans, but membranoproliferative glomerulonephritis and mesangial immune complex deposition were present in clinically asymptomatic sheep.(6)
In a retrospective study of renal tissue from 62 humans with confirmed infective endocarditis, common renal lesions noted were localized infarcts in 31%, and acute glomerulonephritis in 26%. The most common type of glomerulonephritis was vasculitic, without deposition of immunoproteins in glomeruli. Of the renal infarcts, over half were due to septic emboli, mostly in patients infected with Staphylococcus aureus. Acute interstitial nephritis was found in 10% but was more common in biopsy material and seemed attributable to antibiotics. Renal cortical necrosis was found in 10%.(5) In a recent study of long term venous access devices (n=102), it was determined that cultures taken from the inflamed pocket surrounding the implanted port were as reliable as cultures from the catheter tip on removal. The major organisms cultured were coagulase negative Staphylococcus sp., Candida, and Staphylococcus aureus.(2)
JPC Diagnosis:
1. Kidney: Nephritis, cortical, suppurative, acute, multifocal, moderate to severe, with focally extensive coagulative necrosis (infarction), fibrin thrombi and few Gram positive cocci.
2. Kidney: Glomerulonephritis, membranoproliferative, global, multifocal, moderate, with synechiae, glomerular senescence, chronic interstitial nephritis and fibrosis.
3. Ureter: Ureteritis, chronic, diffuse, mild.
Conference Comment:
Numerous specific human glomerular diseases have been characterized in exquisite detail, and an exhaustive review is beyond the scope of these proceedings. In general, glomerular diseases are classified into three broad categories: primary glomerulopathies, systemic diseases with glomerular involvement (i.e. secondary glomerulopathies, e.g. systemic lupus erythematosis [SLE], diabetes mellitus, amyloidosis, bacterial endocarditis, vasculitis), and hereditary disorders (e.g. Alport syndrome, thin basement membrane disease, Fabry disease). Primary glomerulopathies include, among many others, postinfectious glomerulonephritis, rapidly-progressive (crescentic) glomerulonephritis, membranous glomerulopathy, and minimal-change disease.(1) A superb example of crescentic glomerulonephritis was recently examined in WSC 2009-2010, Conference 3, case IV.
Immune mechanisms, including both cell-mediated and antibody-associated, are thought to underlie most primary and many secondary glomerulopathies. The two best-characterized antibody-associated mechanisms of glomerular injury are: 1) antibodies reacting to antigens within the glomerulus; and 2) deposition of circulating antigen-antibody complexes in the glomerulus. In the former, antigens may be intrinsic to the glomerular basement membrane (GBM) proper, or planted in the glomerulus from the circulation. For example, in Goodpasture syndrome, the noncollagenous domain (NC1) of the α3 chain of type IV collagen, intrinsic to the GBM, is the antigen responsible for classic anti-GBM antibody-induced glomerulonephritis. By contrast, myriad antigens, including bacterial products, aggregated immunoglobulins, and nuclear proteins, may be planted in the GBM; antibodies then bind to these antigens, resulting in the formation of immune complexes in situ. Circulating immune complex glomerulonephritis differs from anti-GBM glomerulonephritis in that the immune complexes lack immunological specificity for the glomerulus; rather, they form outside of and localize in the glomerulus due to a variety of physiochemical and hemodynamic factors. The culpable antigens that initiate immune complex formation may be exogenous (e.g. products of infectious agents) or endogenous (e.g. SLE).(1)
Morphologically, glomerulopathies are often evaluated using immunofluorescence microscopy and electron microscopy. Two patterns of immune complex deposition are noted using immunofluorescence: 1) granular, which is characteristic of both circulating immune complex deposition and immune complexes formed in situ against planted antigens; and 2) linear, which is characteristic of classic anti-GBM glomerulonephritis. An understanding of the structures comprising the glomerular capillary wall is essential to properly interpreting the ultrastructure of immune complex glomerulonephritis. The glomerular capillary lumen is bounded by fenestrated endothelial cells, which is further subtended by the GBM, which is composed primarily of type IV collagen, laminin, heparin sulfate, and several other glycoproteins. The GBM has a thick, electron-dense, central lamina densa, and inner and outer, thinner, electron-lucent peripheral layers, the lamina rara interna and lamina rara externa, respectively. The GBM is surrounded by visceral epithelial cells, also known as podocytes, which have foot processes (pedicels) that abut the basement membrane and are separated by 20-30 nm wide filtration slits, each bridged by a thin diaphragm. Finally, mesangial cells, which are contractile, phagocytic cells of mesenchymal origin, are interposed between the glomerular capillaries and support the glomerular tuft. Ultrastructurally, immune complexes are electron-dense deposits which can be localized specifically to one or more of the following sites: subendothelial (i.e. between the fenestrated glomerular capillary endothelium and the GBM), intramembranous (i.e. within the basement membrane), subepithelial (i.e. between the pedicel and GBM), epimembranous (i.e. upon the GBM interposed between adjacent pedicels), or mesangial (i.e. within the mesangial matrix). Localization of immune complex deposition ultrastructually is useful diagnostically, because many glomerulopathies are characterized by immune complex deposition specifically in one or more of these locations.(1)
Membranoproliferative glomerulonephritis, also known as mesangiocapillary glomerulonephritis, may be secondary to other systemic disorders (i.e. secondary MPGN) or idiopathic (i.e. primary MPGN), and is further divided into types I and II based on ultrastructural, immunofluorescent, and pathologic findings. In humans, the majority of MPGN cases are type I, characterized by discrete subendothelial electron-dense deposits ultrastructurally, and C3 deposition in a granular pattern by immunofluorescence. The pathogenesis of type I MPGN involves activation of both the classical and alternative complement pathways; IgG, C1q, and C4 are also often present. In the less common type II MPGN, also known as dense deposit disease, the lamina densa of the GBM contains an irregular, ribbon-like, extremely electron-dense material. Immunofluorescence demonstrates C3 in an irregular granular or linear pattern on either side of the lamina densa within the GBM, but not within the dense deposits. The pathogenesis is thought to involve activation of the alternative pathway; IgG, C1q, and C4 are usually absent.(1)
References:
2. Brouns F, Schuermans A, Verhaegen J, De Wever I, Stas M: Infection assessment of totally implanted long-term venous access devices. J Vasc Access 7:24-28, 2006 (abstract only)
3. Heidel JR, Giddens WE Jr., Boyce JT: Renal pathology of catheterized baboons (Papio cynocephalus). Vet Pathol 18:59-69, 1981 (abstract only)
4. Leary SL, Sheffield WD, Strandberg JD: Immune complex glomerulonephritis in baboons (Papio cynocephalus) with indwelling intravascular catheters. Lab Anim Sci 31:416-420, 1981
5. Majumdar A, Chowdhary S, Ferreira MA, Hammond LA, Howie AJ, Lipkin GW, Littler WA: Renal pathological findings in infective endocarditis. Nephrol Dial Transplant 15:1782-1787, 2000
6. Rao VP, Poutahidis T, Marini RP, Holcombe H, Rogers AB, Fox JG: Renal infarction and immunemediated glomerulonephritis in sheep (Ovis aries) chronically implanted with indwelling catheters. J Am Assoc Lab Anim Sci 45:14-19, 2006