CASE I:
Signalment:
2-week-old, chicken (Gallus gallus)
History:
A 2-week-old specific pathogen free chickens was subcutaneously inoculated with infectious bursal disease (IBD) virus which is classified as a very virulent virus. The present case exhibited ruffled feathers on day 3 postinoculation. The bird showed severe depression and was found dead on day 4 postinoculation.
Gross Pathology:
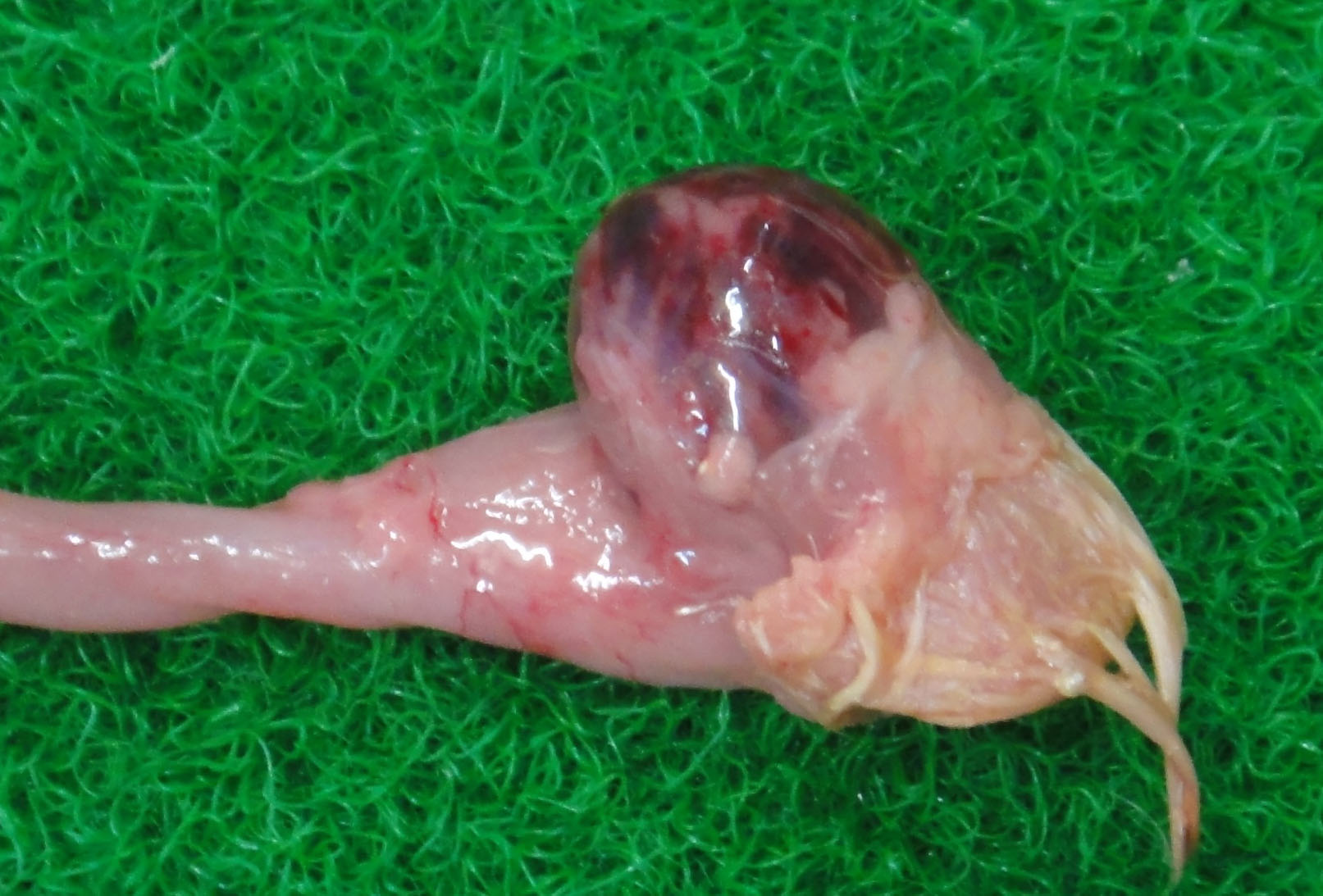
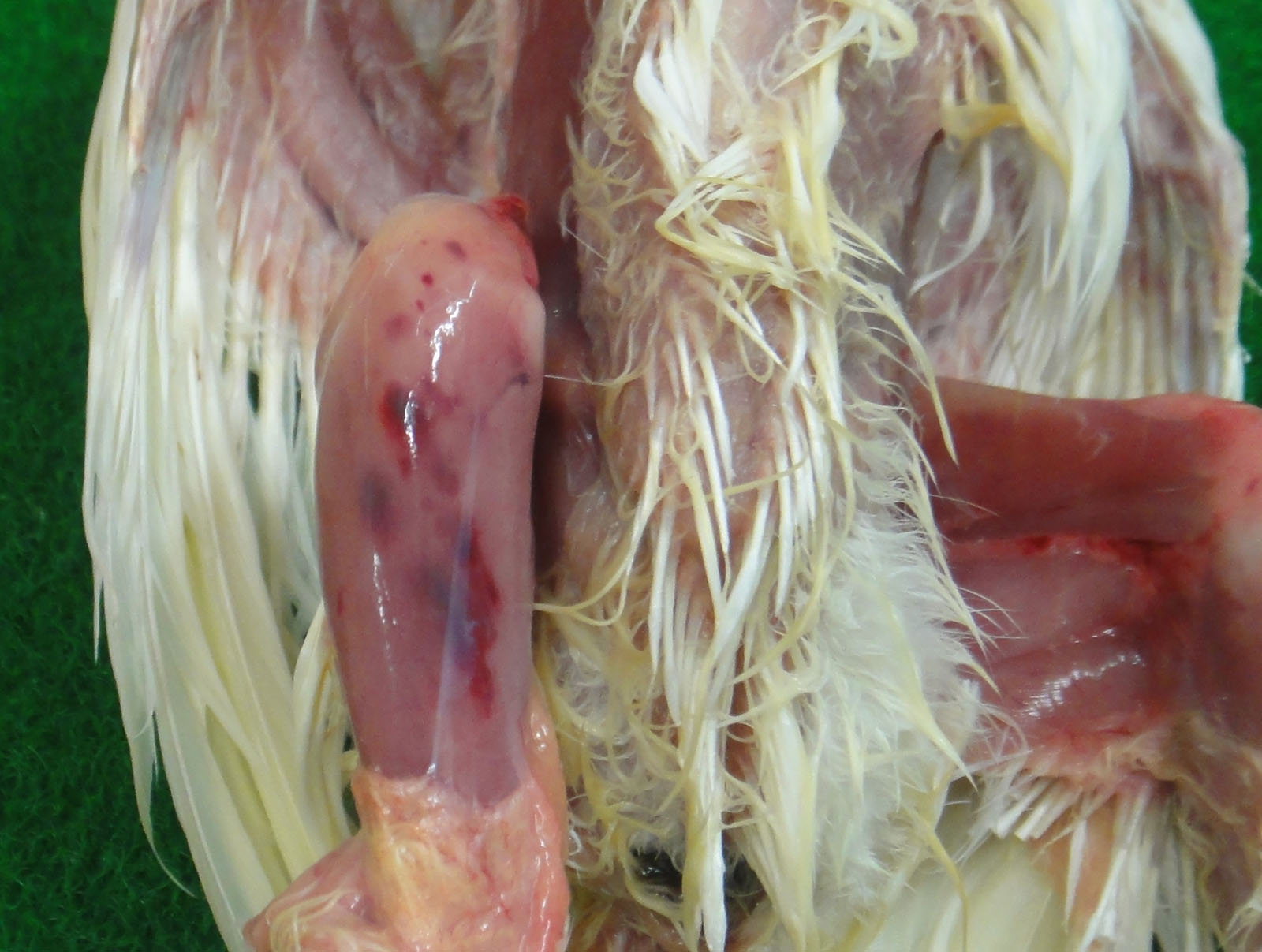
The bursa of Fabricius (BF) was hemorrhagic and slightly enlarged. The muscle of both legs exhibited focal hemorrhages. The hemorrhage was also found at the papillae of the mucus membrane of the proventriculus. The thymus was yellowish, and was severely atrophied. The kidney was pale.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
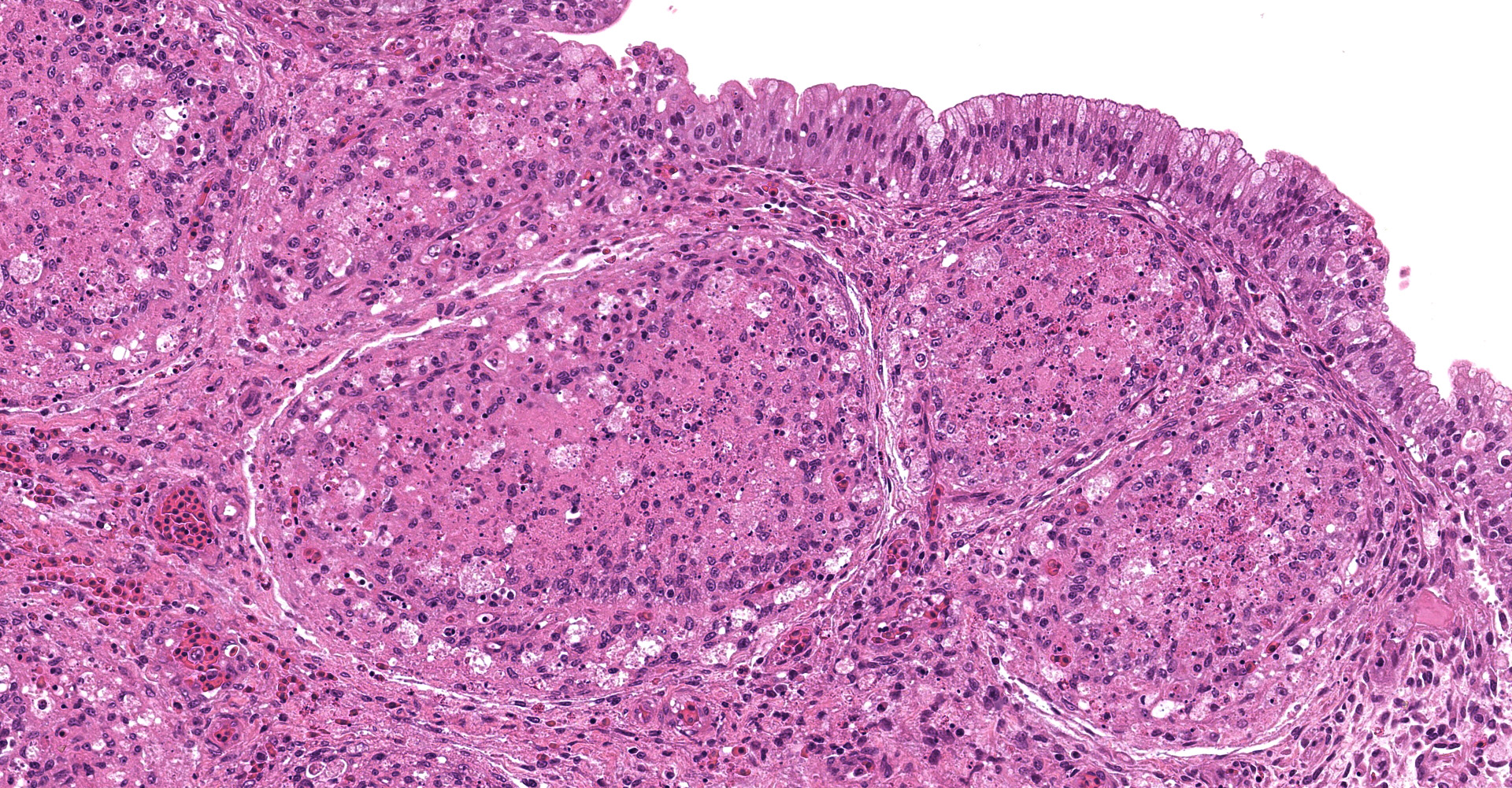
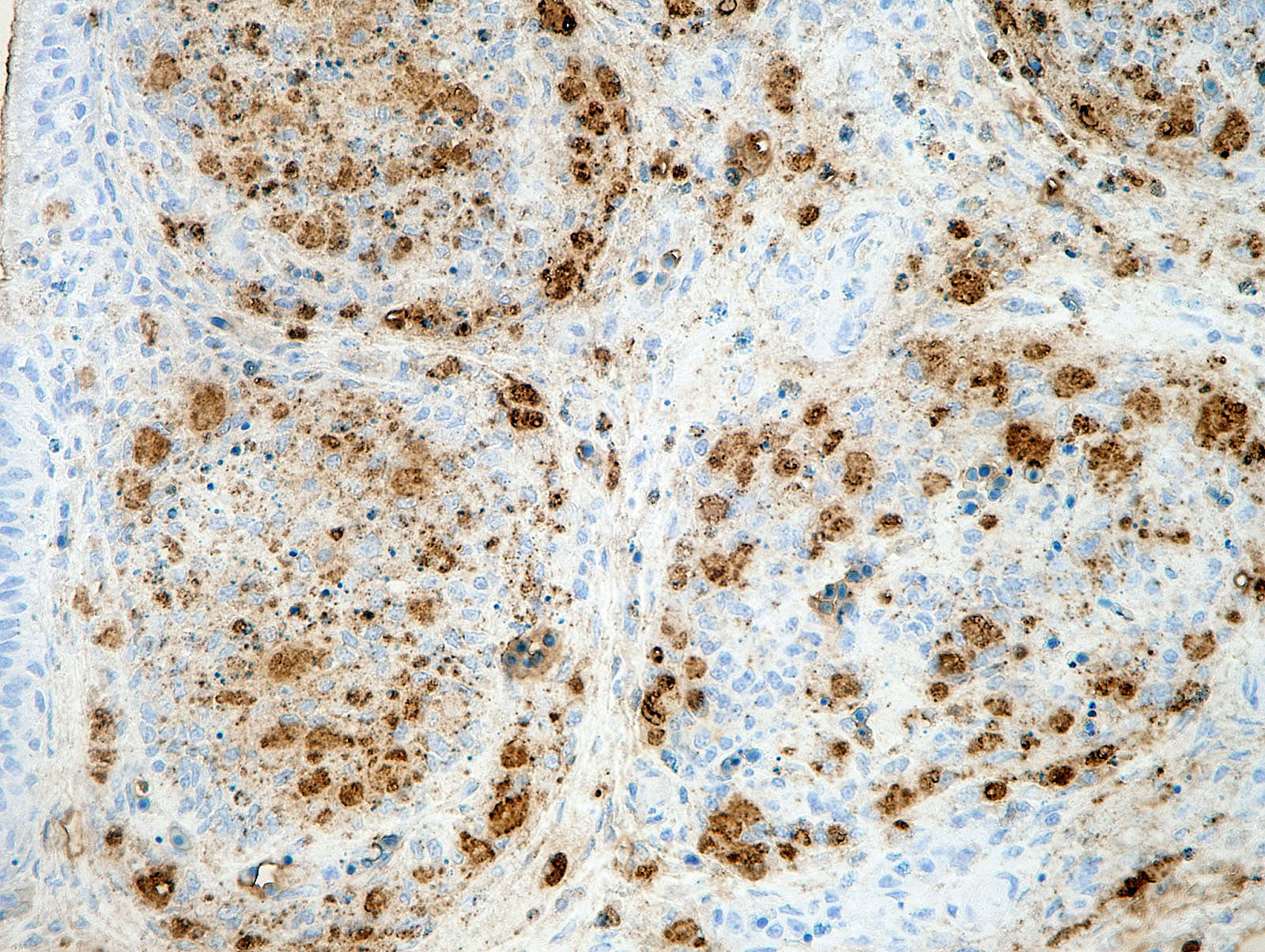
There was a severe depletion of lymphocytes in the BF (karyorrhexis), remaining follicular epithelial cells, and foamy macrophages. Severe hemorrhage and many fibrin thrombi were observed in the interstitium. Bacterial colonies observed in the section were considered to have occurred secondary or postmortem. Viral antigens were detected in cells of the lymphoid follicles and interstitium by immunohistochemistry.
Contributor's Morphologic Diagnoses:
The BF: Diffuse lymphoid necrosis with macrophage infiltration, hemorrhage, fibrin thrombi. (experimental case of IBD)
Contributor's Comment:
IBD is a highly contagious viral disease of chickens.5,12 IBD, also known as Gumboro disease, is caused by IBD virus of the family Birnaviridae.5,12 The genome of IBD virus consists of two segments (A and B) of double-stranded RNA.5,12 The genome encodes 5 viral polypeptides (VP1-5) .5 The large segment A encodes VP2-5; the smaller segment B encodes VP1.5 VP2 and VP3 are the major structural proteins of the virion.5 VP2 is credited with eliciting protective immunity in birds.5
Two serotypes of IBD virus are recognized.5,12 The IBD viruses of serotype 1 are pathogenic for chickens. The serotype 2 viruses are nonpathogenic. The IBD viruses of serotype 1 are generally classified as classical virulent virus, very virulent virus, and antigenically variant virus. The very virulent virus was first identified during the 1980s, and has been detected in many countries.11 Some phylogenetic analyses performed on very virulent viruses showed that they constitute a specific cluster in the phylogenetic tree.11,12,13
The infection of classical virulent viruses is mild or subclinical. However, the impaired growth and immunosuppression as the result of infection can result in the secondary infection and vaccination failure. The very virulent viruses have the ability to cause high mortality rate in affected flocks (i.e. 50-60% mortality in laying hens). The principal macroscopic lesions in chickens exhibiting clinical signs are the edematous swelling, discoloration, and hemorrhage of the BF.5 Hemorrhages in the pectoral muscles and thigh muscles can be observed frequently.5,11,12 This may be caused by a coagulation disorder during the infection.6
Young immature chickens with active lymphocyte development are very susceptible to IBD. Under natural conditions, the infection appears to be via the oral route.5 From the gastrointestinal tract, the virus is transported to other tissues by phagocytic cells, most likely macrophages.5 The bursa of Fabricius is the most susceptible organ.5 IBD virus infection occurs in B lymphocytes in the immature stage with immunoglobulin M expressed on the cell surface, leading to the cytolytic destruction of infected cells.2 Viral antigens can be detected by immunohistochemistry in affected lymphoid follicles.5,9 Some studies indicated that macrophages can be infected with IBD virus.3,4 Although T lymphocytes are considered resistant to infection, the infiltrating T lymphocytes in the lesions may be involved in the pathogenesis.2,5 The pathological lesions can be observed in other lymphoid organs including the thymus, spleen, cecal tonsil, and bone marrow.9 Thymic atrophy may be associated with extensive apoptosis of thymocytes during the acute phase of virus infection.8
Vaccination is popular for protection against IBD.12 Some live vaccine strains can cause mild to severe lesions to the BF, which may provoke immunosuppression and secondary disease.12
Contributing Institution:
National Institute of Animal Health,
National Agriculture and Food Research Organization (NARO)
3-1-5Kannondai, Tsukuba, Ibaraki 3050856, Japan
http://www.naro.affrc.go.jp/english/niah/index.html
JPC
Diagnosis:
Bursa of Fabricius: Lymphoid depletion, follicular, diffuse, severe, with lympho-cytolysis and hemorrhage.
JPC Comment:
First reported in Gumboro, Delaware in 1962, infectious bursal disease virus (IBDV) is a widespread pathogen of worldwide economic relevance. As described by the contributor, IBDV has historically been differentiated into two antigenic groups, serotypes 1 and 2, with serotype 1 strains further differentiated into classic and variant subtypes such as the very virulent strain.7,11 However, significant genotypic and pathotypic variability exists amongst subtypes as the result of frequent mutations and recombination events.7 Consequently, a new genogroup classification scheme based on the hypervariable region of the outer capsid protein VP2 (hvVP2) gene has been proposed, with seven genogroups (G1-G7) identified. The classic, variant, and very virulent strains comprise G1, G2, and G3, respectively.7,11 Strains G4-G7 are composed of a fourth "distinct" strain (G4), a proposed group of recombinant Mexican strains (G5), and divergent strains predominantly identified in Italy (G6) and Australia (G7).11 G4 is composed of several highly divergent strains reported in North and South America, Asia, and Europe with a unique genetic profile that cause subclinical infection with bursal atrophy and immunosuppression. Information in regard to the genetic characteristics of the "distinct" strains of IBDV have historically garnered little attention due to lack of specific clinical signs but have recently come under increased scrutiny as the result of their prevalence and potentially significant economic impact, particularly in South America.11
As noted by the contributor, the virus predominately replicates in IgM positive B lymphocytes, particularly in the bursa of Fabricius (BV), in addition to other lymphoid tissues. The virus causes infected B lymphocytes and adjacent antigen-negative cells to both undergo apoptosis, leading to B cell depletion in the bursa. This process appears to be highly regulated as apoptosis is inhibited by VP5 early in the viral replication cycle, yet VP5 and VP2 induce apoptosis near the end of the replication cycle.1 This phenomenon has also been reported to occur following the administration of vaccines containing live IBDV, with bursal damage occurring 7 to 35 days post vaccination depending on the strain. Notably, the onset of these lesions correlates with the initiation of antibody production. Risk of vaccine induced immunosuppression is a concern, particularly in regard to the potential for degraded humoral responses for other commonly vaccinated diseases, such as Newcastle disease. Conflicting information has been reported in regard to suppression the humoral response to other vaccinations when co-administered with IBDV, with results ranging from no effect on antibody titers despite lesion development in the bursa to significantly decreased antibody levels for three antigens, including Newcastle disease.1
The moderator discussed differentials for bursal atrophy or necrosis, which include chicken anemia virus, chronic Marek's disease, and Newcastle disease virus.
References:
1. Gimeno IM, Schat KA. Virus-Induced Immunosuppression in Chickens. Avian Dis. 2018;62(3):272-285.
2. Hirai K, Calnek BW. In vitro replication of infectious bursal disease virus in established lymphoid cell lines and chicken B lymphocytes. Infect Immun. 1979; 25:964-970.
3. Inoue M, Yamamoto H, Matuo K, et al. Susceptibility of chicken monocytic cell lines to infectious bursal disease virus. J Vet Med Sci. 1992; 54:575?577.
4. Khatri M, Palmquist JM, Cha RM, et al. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res. 2005; 113:44-50.
5. Sharma JM, Kim IJ, Rautenschlein S, et al. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev Comp Immunol. 2000; 24:223-235.
6. Skeeles JK, Slavik M, Beasley JN, et al. An age-related coagulation disorder associated with experimental infection with infectious bursal disease virus. Am J vet Res. 1980; 41:1458-1461.
7. Stoute ST, Jackwood DJ, Crossley BM, Michel LO, Blakey JR. Molecular epidemiology of endemic and very virulent infectious bursal disease virus genogroups in backyard chickens in California, 2009-2017. J Vet Diagn Invest. 2019;31(3):371-377.
8. Tanimura N, Sharma JM. In-situ apoptosis in chickens infected with infectious bursal disease virus. J Comp Pathol. 1998; 118:15-27.
9. Tanimura N, Tsukamoto K, Nakamura K, et al. Association between pathogenicity of infectious bursal disease virus and viral antigen distribution detected by immunohistochemistry. Avian Dis. 1995; 39: 9-20.
10. Tomás G, Marandino A, Techera C, et al. Origin and global spreading of an ancestral lineage of the infectious bursal disease virus. Transbound Emerg Dis. 2020;67(3):1198-1212.
11. van den Berg TP. Acute infectious bursal disease in poultry: a review. Avian Pathol. 2000; 29:175-194.
12. van den Berg TP, Eterradossi N, Toquin D, et al. Infectious bursal disease (Gumboro disease). Rev. sci. tech. Off. int. Epiz. 2000; 19:527-543.
13. Yamaguchi T, Ogawa M, Miyoshi M, et al. Sequence and phylogenetic analyses of highly virulent infectious bursal disease virus. Arch Virol. 1997;142:1441-1458.