Wednesday Slide Conference, Conference 11, Case 4
Signalment:
4-year-old, spayed female, Terrier mixed dog (Canis familiaris).
History:
This dog was presented for diarrhea, swollen paw, an increase respiratory, and a brain tumor. The dog had a long history of neurologic signs, facial tremors, ataxia, eye nystagmus, and myotonic like chomping of jaw. TPLO was done in 2023. CT was performed on 02/2024 and revealed a contrast enhancing mass at the right brainstem cerebral region. Valley Fever titers were negative. The patient was on Prednisone, Gabapentin, Diazepam, and Fluconazole. Patient showed continued deterioration including poor ability to open mouth. Euthanasia was elected.
Gross Pathology:
On thoracic examination, there is no pleural effusion. The left lung lobes are pink to dark red and mottle. The lung lobes are soft, aerated, and slightly wet. The distal 1/3 of the trachea and the mainstem bronchi contain a small amount of white froth. There is no pericardial effusion. The heart is unremarkable.
On abdominal examination, the liver is slightly swollen and congested, and weighs 1.36 kg (5% of the patient’s body weight). The spleen, pancreas, and GI tract is unremarkable.
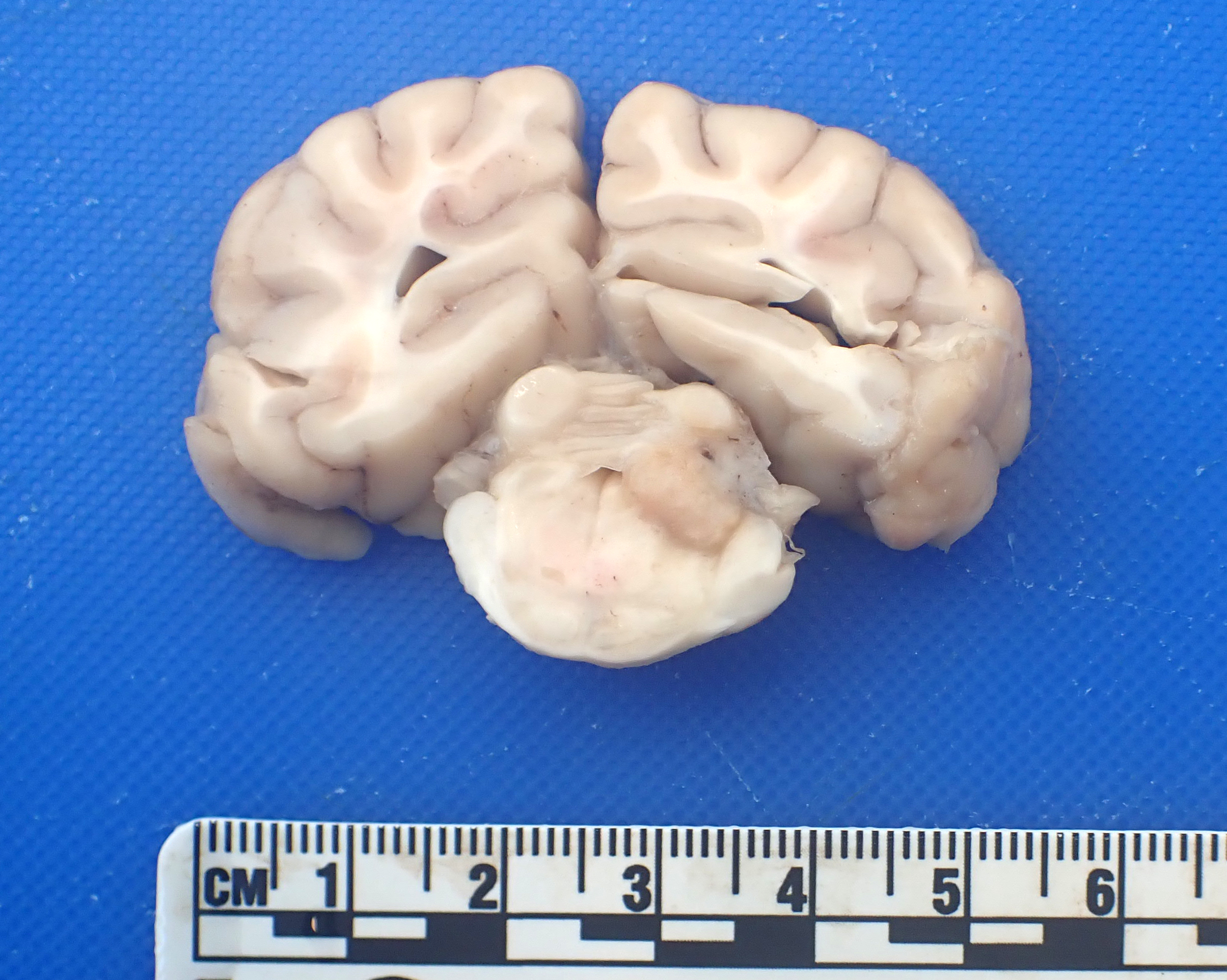
On examination of the head and bran, a 0.7 x 0.4 x 0.3 cm, well-demarcated, expansile, white to tan and firm mass effaces the right-side of caudal cerebellar peduncle and the overlying cerebellar cortex.
Laboratory Results:
Clinical pathology:
Two cytospin preparations from the submitted CSF are reviewed. A 90-cell differential
reveals 31 (34%) non-degenerate neutrophils, 52 (58%) small mononuclear cells, 6 (7%) large mononuclear cells, and 1 (1%) eosinophils. The small mononuclear cells are consistent with small to intermediate sized lymphocytes and have occasional single prominent nucleoli. The lightly basophilic protein background contains many erythrocytes and few polychromatophils. No infectious agents or overt evidence of neoplasia is observed.
Microscopic Description:
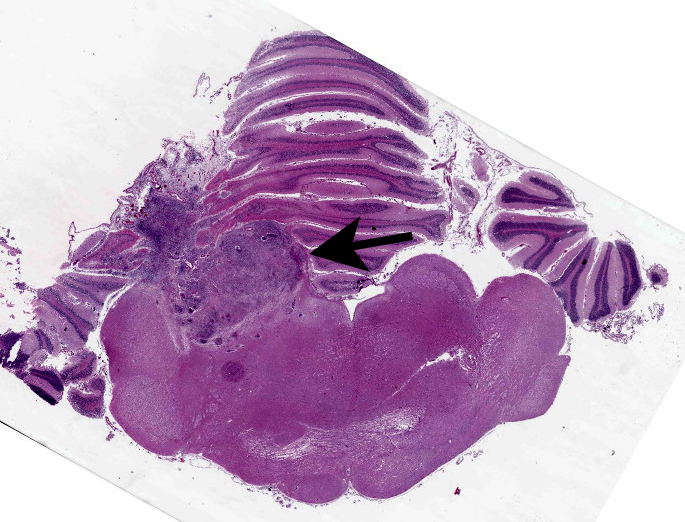
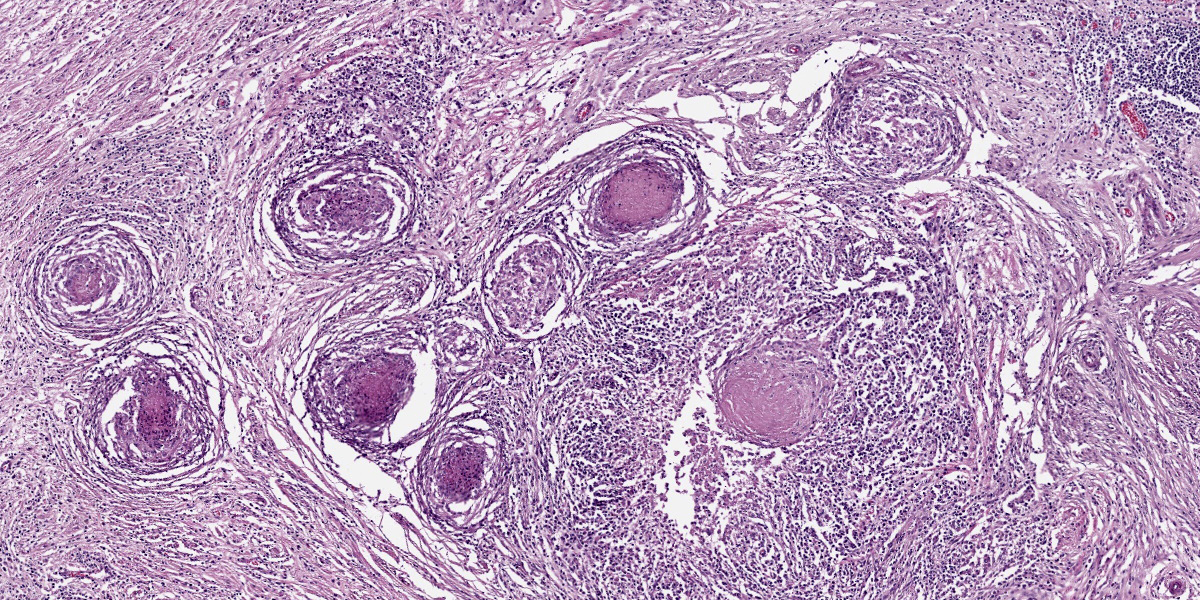
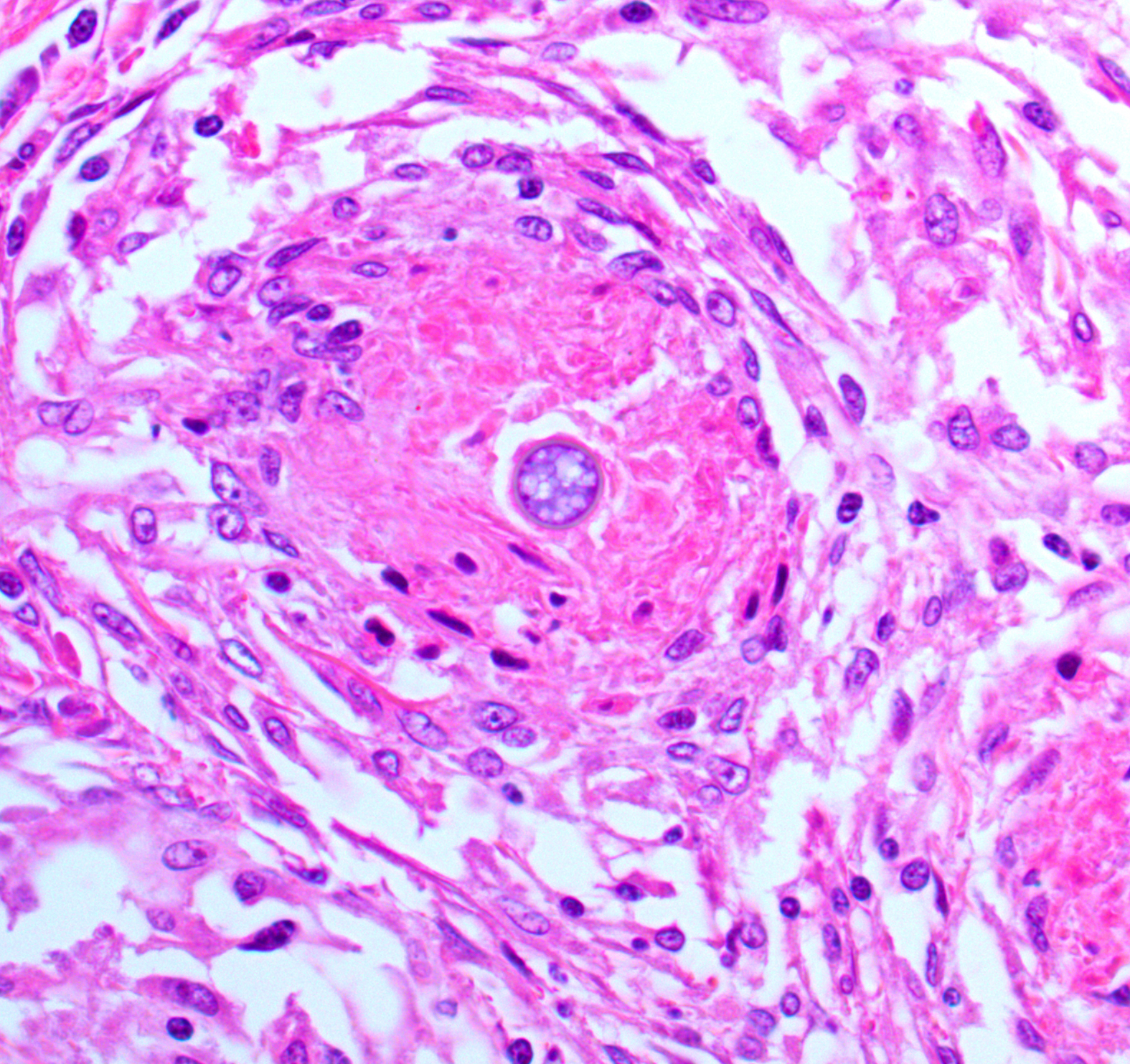
Brain, cerebellum and brainstem: Expanding and effacing the right caudal cerebellar peduncle, right caudal vestibular nucleus and the overlying cerebellar cortical neuroparenchyma are multifocal to coalescing areas of extensive granulomatous inflammation, characterized by central necrosis and large numbers of epithelioid macrophages, which are surrounded by moderate numbers of lymphocytes, plasma cells, and thin band of fibrous connective tissue. The center of granulomas contain small to large amount of eosinophilic cellular debris, karyorrhectic and pyknotic debris, and degenerate leukocytes that occasionally surround a single, 20-30 um in diameter fungal spherule that has a smooth 4um thick, double contoured, hyaline wall containing multiple granular to flocculent, basophilic structures (endospores), consistent with Coccidioides spp. The affected and surrounding neuroparenchyma and meninges are expanded with these inflammatory cells, reactive/ dilated blood vessels, lymphoplasmacytic perivascular cuffings, and a mild to moderate amount of clear space (edema).
Contributor’s Morphologic Diagnosis:
Brain, cerebellum and brainstem: Meningoencephalitis, granulomatous, locally extensive, severe, chronic with intralesional fungal spherules (consistent with Coccidioides sp.)
Contributor’s Comment:
Coccidiomycosis is commonly known as “Valley fever (VF)” and is endemic to human and animals in the southwestern USA (Arizona, California, New Mexico, Nevada) and northern Mexico.3,7,12 Additional areas are Utah, Eastern Washington, Oregon, Central and South Americas.14,15 Coccidiomycosis is caused by soil-dwelling, saprophytic, dimorphic fungi, Coccidioides. Coccidioides was originally described as one species C. immitis, but more recently, genetic analysis has defined two separated species with relative distinct geographical distribution. C. immitis is largely found in California and Eastern Washington State.3,15 All cases in Arizona, Texas and Central and South America are C. posadasii. There have been no distinct differences in the phenotypical behavior of C. immitis and C. posadasii.3,18 Coccidioides requires animal nutrients for growth and their distribution which is largely restricted to areas of human or animal habitation, such as small rodent burrows.3,18 Animal carcasses may serve as a medium for the growth of Coccidioides in the soil.6
Coccidioides spp. grow in soil as a mycelium. Mycelia septate and produce spores known as arthroconidia which are fragile and become airborne with minimal soil disturbance. The infection is almost always through the inhalation of arthroconidia, rarely cutaneous inoculation, which transform into spherules that subsequently divide into endospores. With spherules lysis, the endospores are released and become spherules which result in exponential propagation in affected organs.3,6,12
In humans, 60% of infections are asymptomatic with the remaining 40% having respiratory illnesses. Approximately 1% of total infections are disseminated.12 In dogs, infection most commonly results in self-limiting respiratory tract disease, but disease can be more severe in some dogs which can result in systemic disease.14,15 Large breed, young adult dogs are most at risk. Risk factors are increased for outdoor dogs (roaming areas more than 1 acre) and walking in the desert.4 Boxers, Pointers, Australian Shepherds, Beagles, and Scottish Terriers showed an increased risk of infections as well.11
Disseminated coccidioidomycosis includes cutaneous, osseous, cardiac, ocular, and nervous abnormalities along with other signs of systemic illness.5,9,11 In dogs, CNS involvement typically involves the cerebrum with intracranial lesions often showing focally, or less commonly, as multifocal distinct granulomatous inflammation. Conversely, in human CNS infection, inflammation is more diffuse.2,13,17 Diffuse, bilateral, symmetric lesions of the caudate nuclei and frontal lobes are less common form in dogs, and the median age of intracranial coccidioidomycosis is 7 years.13 The time between infections and evidence of disseminated disease ranges from weeks to several years, and a history respiratory signs might not be present.8,16 Antibody detection is the most sensitive method for VF diagnosis, but it can be falsely negative in approximately 5-10% of cases.17 Therefore, coccidioidomycosis should be considered as a potential cause for chronic illness, respiratory signs, lameness, lymphadenopathy, and nonhealing cutaneous lesions, neurological and cardiac illness within endemic regions.
Coccidioidomycosis occurs less frequently in cats, but their presentation and laboratory abnormalities are similar to dogs including respiratory illness, neutrophilia, monocytosis and hyperglobulinemia. However, cats at diagnosis are typically significantly ill and 60% of cats have disseminated infection, most commonly to the skin, in the endemic regions.1,10
Contributing Institution:
Midwestern University, College of Veterinary Medicine
Diagnostic Pathology Center
5725 West Utopia Rd.
Glendale, AZ 85308
https://clinics.midwestern.edu/animal-health-institute/diagnostic-pathology-center
JPC Diagnosis:
Cerebellum and brainstem: Meningoencephalitis, granulomatous, chronic, focally extensive, severe, with intra-and extracellular fungal spherules.
JPC Comment:
The contributor provides a nice writeup on coccidiomycosis that prompts some interesting questions for this case. The lesion in this case was focal and unilateral, and extended from the leptomeninges through the cerebellar peduncle and into the brainstem.
That this animal reportedly had neurologic deficits attributable to cranial nerve V (motor) and VII deficits aligns with the relative location of these nuclei and the lesion within the brainstem – the focally extensive necrosis of the cerebellum and its role in smoothing motor output likely also played a role, to include the observed nystagmus.
Although the granulomas within the brainstem were an obvious feature on H&E, there were few organisms in histologic section. Even with a GMS or PAS Light Green Stain applied, we noted only 2 granulomas that had an intact spherule. This aligns with the longer clinical course reported by the contributor. Likewise, the lack of free infectious agent (either sequestered in a granuloma and/or low in total number) may have led to a paucity of antigen such that it was below the limit of detection for PCR in this case.
The changes in this case are a classic Th1-response. Participants were quite familiar with Th1 granulomas, though the origin of fibrosis within the brain was less immediately apparent. With chronic antigen stimulation (such as failing to clear Coccidioides), there is predilection for Type-1 macrophages and CD4+ Th1 lymphocytes who in turn produce IFN-γ. IFN-γ is crucial for classical macrophage activation, though this phenotype is notably antifibrotic with cellular arginine being converted to citrulline by nitric oxide synthase 2 which enhances microbicidal activity. In Th2 responses, profibrotic alternatively activated macrophages instead convert arginine to ornithine and eventually proline (a collagen precursor). While the normal brain parenchyma lacks fibroblasts to create fibrous tissue, there are two potential sources to strategically acquire them. In this case, the extension of inflammation through to the meninges (and associated meningeal fibroblasts) is the most likely explanation. Alternatively, differentiation of perivascular fibroblasts (from pericytes) is another possibility that may explain some of the fibrosis deeper in tissue section.7 The disruption of the blood-CNS barrier may also play a role.7
References:
- Arbona N, Butkiewicz CD, Keyes M, et al. Clinical features of cats diagnosed with coccidioidomycosis in Arizona, 2004-2018. J Feline Med Surg. 2020 22(2):129-137.
- Bentley RT, Heng HG, Thompson C, et al. Magnetic resonance imaging features and outcome for solitary central nervous system Coccidioides granulomas I 11 dogs and cats. Vet Radiol Ultrasound. 2015 56(5):520-530.
- Brown J, Benedict K, Park BJ, et al. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013 25(5):185-197.
- Butkiewicz CD, Shubitz LF, Dial SM. Risk factors associated with Coccidioides infection in dogs. J Am Vet Med Assoc. 2005;226(11):1851-1854.
- Davidson AP, Shubitz AF, Alcott CJ, et al. Selected Clinical Features of Coccidioidomycosis in Dogs, Medical Mycology, 2019, S67–S75.
- Del Rocio Reyes-Montes M, Ameyali Perez-Huitron M, Luis Ocana-Monroy J, et al. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infectious Diseases. 2016;16:550.
- Fehlberg CR, Lee JK. Fibrosis in the central nervous system: from the meninges to the vasculature. Cell Tissue Res. 2022 Mar;387(3):351-360.
- Galgiani JN, Ampel NM, Blair JE, et al. Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin Infect Dis. 2016 15;63(6):e112-146.
- Graupmann-Kuzma A, Valentine BA, Shubitz LF, et al. Coccidioidomycosis in dogs and cats: a review. J Am Vet Med Assoc. 2008;44(5):226-235.
- Greene RT, Troy GC. Coccidioidomycosis in 48 cats: a retrospective study (1984-1993). J Vet Intern Med. 1995, 9(2):86-91.
- Johnson LR, et al., Clinical, clinicopathologic, and radiographic findings in dogs with coccidioidomycosis: 24 cases (1995-2000). J Am Vet Med Assoc, 2003. 222(4):461-466.
- Johnson RH, Sharma R, Kuran R, et al. Coccidioidomycosis: a review. J Investig Med. 2021, 69(2):316-323.
- Kelley AJ, Stainback LB, Knowles KE, et al. Clinical characteristics, magnetic resonance imaging features, treatment, and outcome for presumed intracranial coccidioidomycosis in 45 dogs (2009-2019). J Vet Intern Med. 2021, 35(5):2222-2231.
- Lockhart SR, McCotter OZ, Chiller TM. Emerging Fungal Infections in the Pacific Northwest: The Unrecognized Burden and Geographic Range of Cryptococcus gattii and Coccidioides immitis. Microbiol Spectr. 2016. 4(3).
- Meisner J, Clifford WR, Wohrle RD, et al. Soil and climactic predictors of canine coccidioidomycosis seroprevalence in Washington State: An ecological cross-sectional study. Transbound Emerg Dis. 2019. 66(5):2134-2142.
- Shubitz LF, Butkiewicz CD, Dial SM, et al. Incidence of Coccidioides infection among dogs residing in a region in which the organism is endemic. J Am Vet Med Assoc. 2005;226(11):1846-1850.
- Spoor E, Stainback L, Plummer S, et al. A novel form of intracranial coccidioidomycosis is present in dogs and exhibits characteristic clinical and magnetic resonance imaging findings. Vet Radiol Ultrasound. 2019. 60(1):47-55.
- Taylor JW, Barker BM. The endozoan, small mammal reservoir hypothesis and the life cycle of Coccidioides species. Med Mycol. 2019, 1(57):S16-S20.