Signalment:
2-year-old, female, Sprague-Dawley rat (
Rattus norvegicus).Animal was part of the vehicle control group of a two-year gavage carcinogenicity study. A scheduled terminal necropsy was performed on day 731 of the test.
Gross Description:
The terminal body weight was 339.1 grams. The submitted tissues correlate with
two masses located in the right inguinal region. These masses were dark red to tan, lobulated, and firm. Other necropsy findings included an enlarged adrenal cortex, an enlarged pituitary compressing the ventral brain, and dark red foci throughout the liver.
Histopathologic Description:
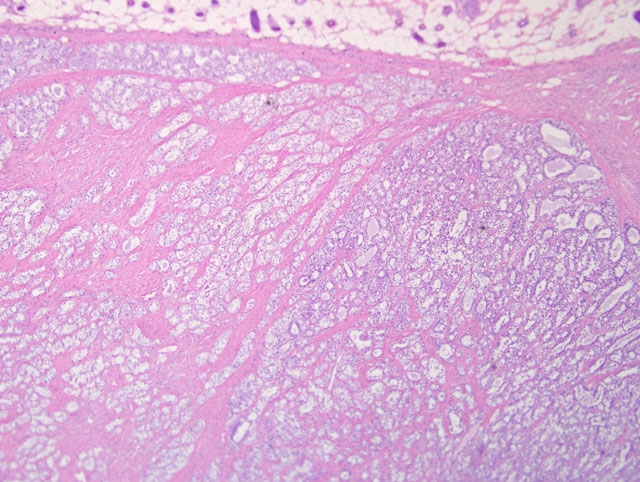
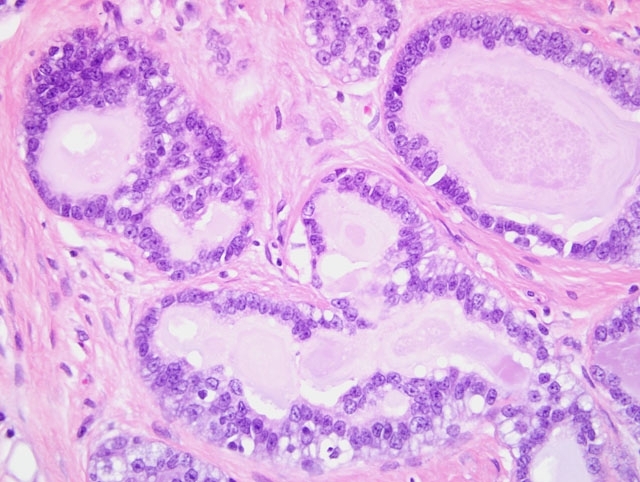
Haired skin and mammary gland: Histologically, there is an expansile, non-encapsulated but well-delineated mass within the subcutis, which compresses adjacent tissues. The mass is multilobulated with varying islands of epithelial cells separated by thick bands of dense fibrous connective tissue. Thin bands of connective tissue extend into some islands forming a trabecular pattern which separate the epithelial cells into small, single-cell lined acini and tubules while other islands have scant amounts of fibrous connective tissue and contain more dense accumulations and pilings of atypical epithelial cells which compress adjacent lobules. The epithelial cells within the trabeculae of connective tissue are well differentiated with small, dark nuclei and vacuolated cytoplasm, and lumen frequently contains basophilic secretory material within the lumen. The fibrous connective tissue is also composed of well-differentiated cells with abundant streaming eosinophilic cytoplasm and small, dark nuclei. The atypical epithelial components which contain more densely cellular epithelial cells and less connective tissue also contain large ectatic pools of basophilic secretory material which are occasionally mineralized. These atypical epithelial cells exhibit mild to moderate anisocytosis with abundant basophilic to vacuolated cytoplasm and large, round, vesicular nuclei with one or two prominent magenta nucleoli. Mitotic figures are fewer than 1 per high magnification field. There is a minimal infiltrate of neutrophils scattered throughout the mass. Well developed blood vessels are common throughout the mass although clear evidence of metastatic invasion is not observed.
Morphologic Diagnosis:
Mammary glands: Adenocarcinoma arising in a fibroadenoma.
Condition:
Adenocarcinoma arising in a fibroadenoma
Contributor Comment:
Other significant findings in this animal included: adrenal gland cortical cell adenoma, contralateral cortical atrophy, cystic hemorrhagic degeneration; liver angiectasis and biliary ductular hyperplasia; pituitary gland pars distalis adenoma; ovaries atrophy; thymus atrophy; and uterus endometrial fibrosis. In this particular two-year study the incidence of mammary tumors in 100 control group females included 28 fibroadenomas, 3 adenomas, 25 adenocarcinomas, and 7 adenocarcinomas arising in fibroadenomas.
Mammary tumors are extremely common spontaneous lesions in aging rats although the incidence and type of tumor varies considerably from one rat strain to the next. Within the F344 strain, fibroadenomas are reported in up to 60% of females surviving the length of a two-year study.(2) The incidence of this same tumor in Wistar rats is 45% and in Sprague-Dawley rats ranges from 24-68% in females at the end of a two-year study.(4,5) In most of the common rat strains, adenocarcinomas are considerably less common than fibroadenomas. In toxicologic studies, this variation in tumor incidences between strains demonstrates the importance of relevant controls when assessing whether a tumor is test-article related or an incidental finding. Furthermore, this variation also suggests that there are important pathogenic distinctions between the strains to consider when using data from laboratory animals to evaluate human health risks. It has long been established that the quantity and ratio of estrogen to prolactin have a profound influence on the biological behavior of these tumors in rats.(5) In addition to xenobiotics, there are numerous environmental factors such as diet, pregnancy status, and housing conditions which can further influence these tumor incidences.
The criteria for classification of mammary tumors in the rat have been well described.(1) Additional clarification and refinement of diagnostic criteria have been accomplished to enhance uniformity of terminology among pathologists.(3) This harmonization is an important and ongoing process that is essential for accurate risk assessment by the various regulatory agencies throughout the world. In conjunction with this harmonization, continued monitoring of the incidence of spontaneous tumors in these various strains provides the opportunity for recognition of important biological shifts in tumor behavior over time.
JPC Diagnosis:
Haired skin, mammary gland: Mammary fibroadenoma, with atypia.
Conference Comment:
Conference participants carefully considered the contributors diagnosis of adenocarcinoma arising in fibroadenoma, which was included in the differential diagnosis, along with lobular hyperplasia, fibroadenoma, and fibroadenoma with atypia. Consistent with the contributors description, the microscopic sections consist of haired skin and mammary gland containing an unencapsulated, well-demarcated, expansile mass composed of epithelial cells arranged in well-differentiated acini and tubules and separated by variable amounts of dense fibrous connective tissue stroma. Acini and tubules are generally lined by a single layer of neoplastic epithelial cells. Epithelial cells are cuboidal and have moderate amounts of vacuolated cytoplasm and round to oval, finely stippled nuclei and indistinct nucleoli. There are few mitotic figures. The fibrocollagenous stroma is composed of well-differentiated spindled cells with eosinophilic fibrillar cytoplasm and a small, elongate nuclei. While some participants identified scattered mitoses and areas of epithelial atypia in the neoplasm, including piling up of epithelial cells in clusters of acini, based on the sections available for evaluation, participants did not observe features indicative of adenocarcinoma, such as invasion through the basement membrane, desmoplasia, necrosis, high mitotic rate, clumped chromatin, bizarre mitoses, squamous metaplasia, or the histologic patterns associated with mammary adenocarcinoma in the rat (i.e. comedo, cribriform, or papillary).3 That said, this lesion represents the difficulty in examination of numerous sections of a neoplasm, and the initial tissue sections evaluated by the contributor may well have contained areas demonstrating convincing adenocarcinoma within mammary fibroadenoma.
The chart below summarizes key histologic features of the differentials as indicated in the Standardized System of Nomenclature and Diagnostic Criteria (SSNDC) Guide:(3)
| Lobular hyperplasia | Lobular enlargement by histologically normal, hyperplastic alveoli
Single layer of alveolar epithelium |
| Atypical hyperplasia | Focal irregular epithelial proliferation with cellular atypia and/or pleomorphism
Formation of epithelial papillae, arches, nests or plaques projecting into the lumen |
| Adenoma | Well-circumscribed proliferation of clusters of tubuloacinar structures on a scanty collagenous stroma
Alveoli lined by a single layer of epithelium with a small nucleus, one nucleolus and cytoplasm that is often vacuolated
Papillary or cystic papillary patterns |
| Fibroadenoma | Two morphologically distinct cell populations -¦ areas of atypia/cellular pleomorphism
- Epithelium single layer, uniform, with or without lipid vacuoles forming tubuloacinar structures or cysts
- Fibrous connective tissue of variable density coursing within and between lobules with few fibrocytes
|
| Adenocarcinoma | Uniform epithelial cells varying from one to many cells thick; have a central round nucleus; clumped chromatin; one nucleolus and many mitoses
May or may not be invasive
Patterns include papillary, tubular, cribiform, or comedo |
| Adenocarcinoma arising in a fibroadenoma | Histologic pattern of adenocarcinoma
Variable histologic pattern of fibroadenoma component |
References:
1. Boorman GA, Wilson JT, van Zwieten MJ, Eustis SL. Mammary gland. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds.
Pathology of the Fischer Rat. San Diego, CA: San Diego Academic Press; 1990:298-305.
2. Haseman JK, Hailey JR, and Morris RW. Spontaneous neoplasm incidences in Fischer344 rats and B6C3F mice in two-year carcinogenicity studies: a national toxicology program update.
Toxicol Pathol. 1998;26(3): 428-41.
3. Mann PC, Boorman GA, Lollini LO, McMartin DN, Goodman DG. Proliferative lesion of the mammary gland in rats. In:
Guides for Toxicologic Pathology; accessed at
http://www.toxpath.org/nomen/index.htm, 11 August 2010. Washington D.C.: Society of Toxicologic Pathologists/American Registry of Pathology/The Armed Forces Institute of Pathology; 1996
4. Poteracki J, Wash KM. Spontaneous neoplasms in control Wistar rats: a comparison review.
Toxicol Sci. 1998;45(1):1-8.
5. Van Zwieten MJ, Hogenesch H, Majka JA, Boorman GA. Non-neoplastic and neoplastic lesions of the mammary glands. In: Mohr U, Dungworth DL, Capen CC eds.
Pathobiology of the Aging Rat Volume 2. Washington D.C.: ILSI Press; 1994:459-475.