Signalment:
Gross Description:
Histopathologic Description:
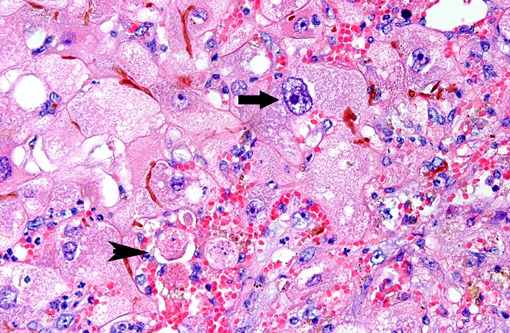
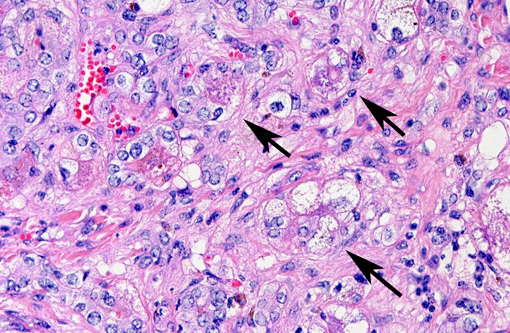
Predominantly in centrilobular regions, numerous hepatocytes show marked hepatocellular polyploidy and hepatocellular hypertrophy (megalocytosis) with marked nuclear pleomorphism often with cytoplasmic hypereosinophilia, foamy cytoplasm and multiple nuclei. Hepatocellular nuclei are often swollen, containing up to three large nucleoli and occasional mitotic figures and cytoplasmic inclusion. There are frequent intercellular bile plugs in bile canaliculi and swelling and proliferation of Kupffer cells containing bile and lipofuscin pigment. Various amounts of bile and lipofuscin pigment are also present in the cytoplasm of hepatocytes.Â
There is multifocal, predominantly centrilobular, hyperemia with absence of hepatocytes, hepatocellular swelling and eosinophilia and karyopyknosis and karyorrhexis (hepatocellular necrosis) with associated infiltration of neutrophils, macrophages and swelling and proliferation of endothelial cells.Â
There are diffuse, randomly scattered islands of smaller hepatocytes arranged in broadened cords of liver cells (nodular hyperplasia), with hepatocytes frequently containing intracytoplasmatic vacuoles of varying size with sharp edges (hepatocellular lipidosis) and single apoptotic hepatocytes.Â
There is increased sinusoidal cellularity with infiltration of various numbers of neutrophils, histiocytes and lymphocytes.Â
The liver capsule shows moderate diffuse fibrosis and hypertrophic mesothelial cells and there is multifocal to coalescing mild lymphohistiocytic and neutrophilic sub-capsular infiltration.Â
Morphologic Diagnosis:
Lab Results:
| -Lymphocytes | 7.7 | â | (25-70 %) |
| -Granulocytes | 88.7 | â | (30-65 %) |
| -ALP | 647 | â | (97-269 U/l 37ËC) |
| -CPK | 391 | â | (<268 U/l 37ËC) |
| -Creatinine | 197 | â | (116-180 μmol/l) |
| -Total bilirubin | 215 | ââ | (<34 μmol/l) |
| -GOT | 600 | â | (22-488 U/l 37ËC) |
| -LDH | 828 | â | (162-412 U/l 37ËC) |
| -GGT | 914 | ââ | (16-56 U/l 37ËC) |
Condition:
Contributor Comment:
Widespread portal fibrosis, bile duct proliferation, and megalocytosis are often described as characteristic lesions observed in the liver of horses poisoned by plant-derived pyrrolizidine alkaloids (PA) 1,4,6,7. These chemicals have been found in various plant species widely distributed in the world, e.g. in genera Senecio, Crotalaria, Heliotropium, Amsinckia, Cynoglossum, Echium, and Trichodesma (1,6). In The Netherlands, predominantly Senecio ssp. (e.g. Jakobskruiskruid, Senecio jacobaea) from theAsteraceae family, are of increasing epidemiologic importance(4).Â
Ingested PAs are converted to pyrrolic esters by hepatic cytochrome p450 enzymes which mediates the N-oxidation of the esters. These esters are alkylating agents, which react with cytosolic and nuclear proteins and nucleic acids(6). Dissociation of the alkylation products may result in the formation of new alkylating agents, which may cause cellular damage to persist after ingestion of the alkaloid has ceased.Â
Cytochrome p450 can also mediate a two-step hydroxylation of necine bases at C3 and C8 positions which is followed by spontaneous dehydration to the highly reactive dehydro-pyrrolizidine (DHP). The toxic DHP metabolites are electrophilic and can bind to proteins and nucleic acids at guanine and adenine residues, and this DNA-binding activity is responsible for their genotoxicity(6).Â
There are three common pathological expressions of PA poisoning (6):
1. Acute periacinar zonal necrosis, occurs after ingestion of large amounts of PA (naturally occurring outbreaks are rare).Â
2. Hepatic atrophy with formation of regenerative nodules and characteristic pattern of hepatocellular megalocytosis, occurs after phasic (usually seasonal) repetitive exposure; this is the most common expression of field exposure to PAs.Â
3. Firm, atrophic livers without nodular regeneration, due to prolonged exposure.Â
Several studies on horses with experimental PA intoxication revealed that development of megalocytosis, as well as advanced fibrosis and bile duct proliferation, occurs in rather late/ chronic stages (up to 6 month) and/or due to prolonged low dose intoxications(4,6). Furthermore, clinical signs of toxicosis generally do not appear until weeks to months after onset of plant consumption and as soon as marked clinical signs occur it was found that in most cases the pathological damage is irreversible(2,4). The kinetics of conversion of alkaloids to pyrroles, and the chemical nature of these products, varies not only with plant species, but also with animal species, age and sex, and with the metabolic rate of the target cells(4,6). For an individual PA, toxicity depends on the amount of alkaloid that can be converted to reactive metabolites, the rate of cytochrome p450-mediated generation of DHP, and the efficiency of the detoxification pathway(6). Hence, the fact that in this case only one individual of the herd showed clinical symptoms of hepatic failure is feasible. In addition to this, estimating the onset of exposure to the toxin is very difficult.Â
One of the most characteristic effects of chronic exposure to toxic pyrroles is the induction of nuclear and cytoplasmic gigantism (megalocytosis). This effect is most likely due to an antimitotic effect with continued DNA synthesis6. Continued nucleoprotein synthesis, coupled with mitotic inhibition, probably accounts for the great increase in size of the nucleus and cytoplasm(6). Some enlarged nuclei have cytoplasmic invaginations that can be misinterpreted as intranuclear inclusions(6). Megalocytic cells can be up to 20 times normal size, however, many hepatocytes in an affected liver do not become megalocytic. Those that are completely inhibited do not replicate DNA at all, whereas those that are more resistant can replicate more normally and give rise to nodular populations
of smaller, more normal hepatocytes. When hepatocytes are lost faster than they can be replaced, the liver can become atrophic, but the atrophy can be compensated to various degrees by megalocytosis and regenerative nodules(6). Regenerative capacity might be, amongst others, due to species specific differences, because cattle more often show regenerative nodules than horses(6).Â
Concurrently, there is usually fibroplasia and proliferation of the bile ducts. Proliferation of bile duct epithelial cells is largely explained by their unspecific propensity to response to regenerative stimuli that prevail when liver mass is inadequate. The amount of fibroplasia varies with species and exposure, typically it is minimal in sheep, moderate in horses and may be marked in cattle(6).Â
Horses are more likely to manifest signs of hepatic encephalopathy than other species, characterized by head pressing and compulsive walking, the latter of which also occurred in this patient. Although it could not be found in this case, pulmonary emphysema is described as an outstanding feature of pulmonary manifestation of certain PA intoxications in horses. The pulmonary lesions include severe vascular engorgement and edema, and diffuse fibrosis of alveolar and interlobular septa with patchy epithelialization(6).Â
Other alkylating agents such as nitrosamine and aflatoxins can also result in portal fibrosis, bile duct proliferation and sometimes megalocytosis(6,7). Due to lacking information about the quality of the patients diet, chronic hepatic toxicosis from e.g. aflatoxins cannot be completely excluded.Â
Although the histological lesions reveal characteristics of a chronic hepatitis, pathologists often do not include the term hepatitis in the morphological diagnosis of this toxic liver condition. This seems to reflect an inconsistency in the use of the term hepatinflammatory reaction.Â
JPC Diagnosis:
Conference Comment:
The moderator pointed out that in cases of hepatocellular nodular regeneration, regenerated hepatocytes develop compensatory enzymes to better accommodate repeat toxin exposure. This is recognized histologically in cases such as chronic copper toxicity, where regenerative nodules are often devoid of copper.Â
In this case, biliary hyperplasia can be characterized using the new scheme, as described in the previous case, as ductal reaction type 2A. The incipient metaplastic change in the bile ducts is the result of ductal formation by hepatocytes, which lose cytoplasmic eosinophilia and form lumens. . Interestingly, the hepatocytes dedifferentiate to a more immature phenotype in response to chronic exposure to free bile acids secondary to severe cholestasis (3).Â
References:
2. Curran JM, Sutherland RJ & Peet RL: A screening test for subclinical liver disease in horses affected by pyrrolizidine alkaloid toxicosis. Aust Vet J 74:236-240, 1996.Â
3. Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011; 458:251259
4. Mendel VW, Witt MR, Gitchell BS, Gribble DN, Rogers QR, Segall HJ & Knight HD: Pyrrolizidine alkaloid-induced liver disease in horses: An early diagnosis. Am J Vet Res 49(4):572-578, 1988.Â
5. Olsman AFS & Sloet van Oldruitenborgh-Oosterbaan: Primary liver diseases in horses [Article in Dutch]. Tijdschr Diergeneeskd 129:510-511, 2004.Â
6. Stalker, MJ & Hayes, MA. Liver and biliary system. In: Pathology of Domestic Animals, Volume 2, 5th ed., KVF Jubb, PG Kennedy, and N Palmer, eds., Saunders Elsevier; 2007; pp. 373-376.Â
7. Stegelmeier BL, Gardner DR, James LF & Molyneux RJ: Pyrrole detection and the pathologic progression of Cynoglossum officinale (houndtongue) poisoning in horses. J Vet Diagn Invest 8:81-90, 1996.Â