Signalment:
Gross Description:
Pulmonary: The lungs are mottled tan to medium red. Multiple nodules are present, and the tracheobronchial lymph nodes are markedly enlarged. The right cranial lung lobe has a large, firm to soft, smooth, tan to white mass that measures 2.5 x 2.5 x 1 cm. Within the left cranial lung lobe, at the junction between the cranial and caudal subsets, there is a focal, firm, 2 x 2 x 1 cm nodule. Smaller nodules are present at the distal aspect of the left cranial lung lobe that measure up to 1 cm in diameter. A large nodule is present with the accessory lobe that measures 2.5 x 1.3 by 1 cm.
Hemolymphatic: The mediastinal and tracheobronchial lymph nodes are markedly enlarged. All are soft to firm and pale tan to white. The largest of the mediastinal lymph nodes measures 1.7 x 2 x 1 cm and the tracheobronchial lymph nodes measure up to 1.5 x 1.0 x 1.0 cm.
Internal: In the omentum adjacent to the pancreas, there is a soft, smooth, tan to white mass that measures 1.6 x 1.6 x 1.3 cm. The regional omentum is hyperemic and thickened.
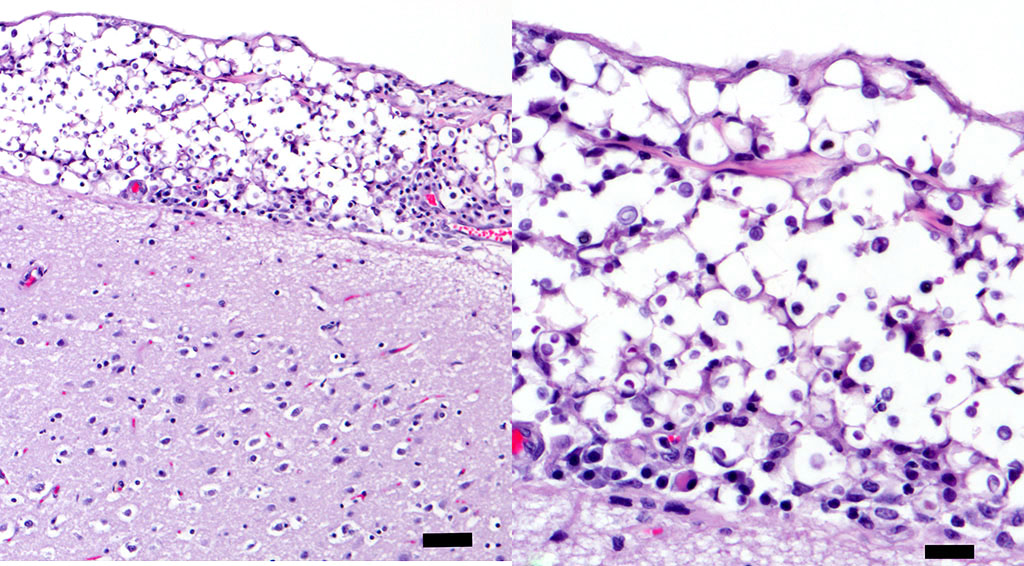
Histopathologic Description:
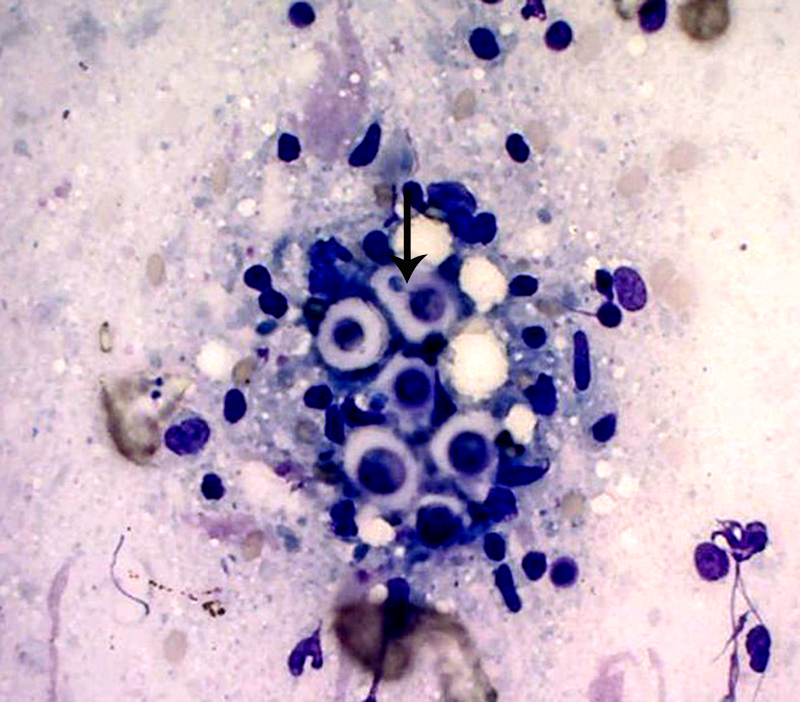
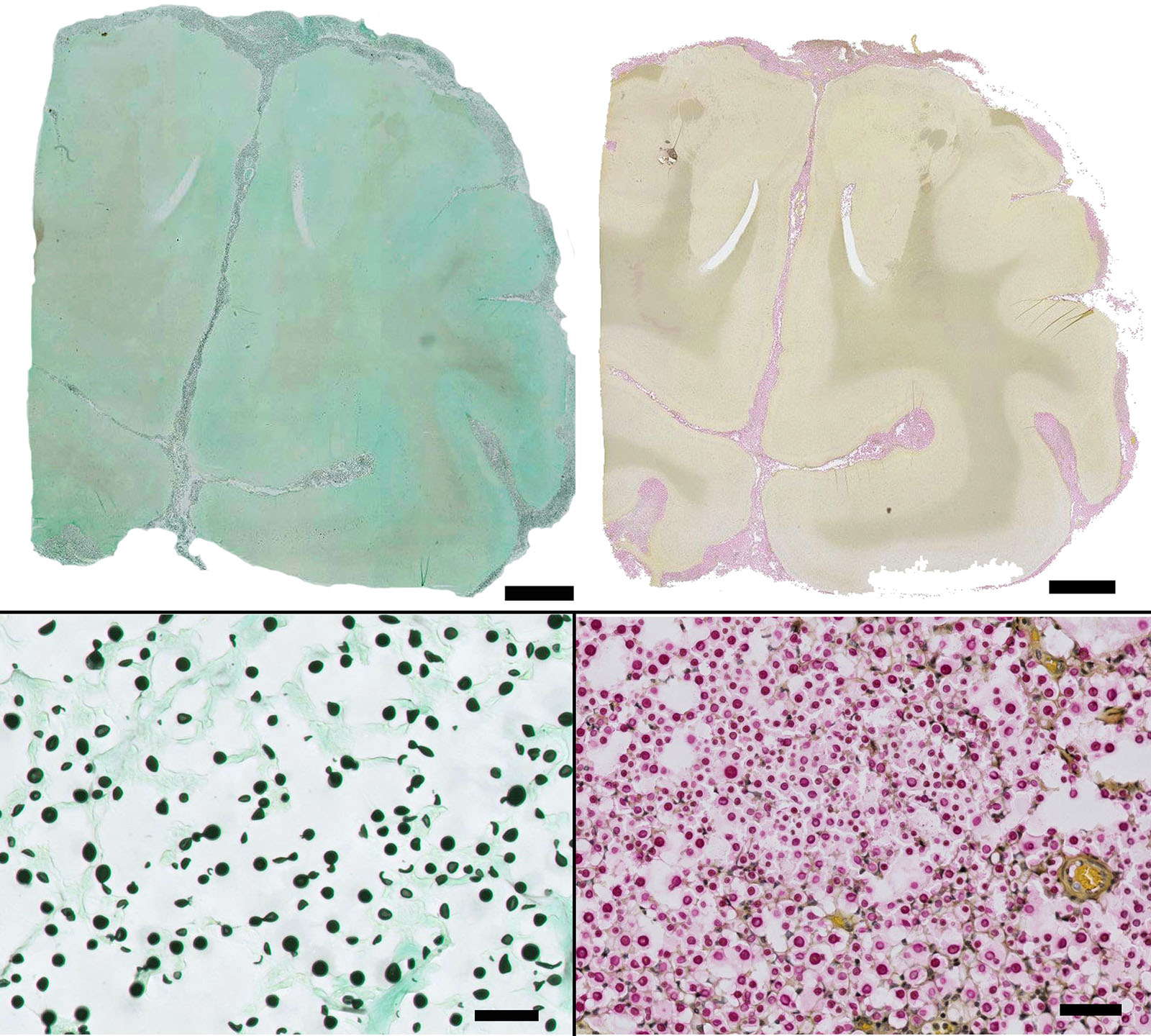
Special stains: Gomori methenamine silver (GMS) and mucicarmine reveal positive staining of yeasts and highlights narrow based budding. The capsule is light pink with the mucicarmine stain.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Pathogenesis is dependent upon the amount of organism present upon exposure, virulence of the strain and immune status of the host. Cryptococcus has been called a sugar-coated killer with designer genes,5 due to its virulence factors.5 The four main virulence factors include the ability to grow at 37 degrees C, its polysaccharide capsule, melanin production and secretion of degradative enzymes.2,5 The polysaccharide capsule provides protection from the environment and host, 5 is comprised of negatively charged glucuronoxylomannan and enlarges after infection of the host.5 The capsule is chemotactic for neutrophils, however, inhibits phagocytosis via its negative charge and blocks the antibody Fc receptor from communicating with host phagocytes. It interferes with leukocyte migration, can deplete complement and inhibits T cell responses. Resolution of infection in immunocompetent animals requires a T-helper cell 1 (Th1) pattern of cytokine and lymphocyte-mediated adaptive immune response.2,4 Tumor necrosis factor, IL-12, IL-18, GM-CSF and IF-? among others play a role in host defense.2 Humoral factors are thought to aid in clearance via antibodies and complement.2 Melanin or a melanin-like compounds are produced from diphenolic compounds via the enzyme laccase (phenoloxidase)2,5 which help protect the organism from oxidative damage7 and may modulate the host immunoinflammatory response.3 Interestingly, rare Cryptococcus spp without a capsule can be easily phagocytized and incite a strong granulomatous response.3
Yeasts were more prominent than inflammation in the majority of the sample. Cryptococcus infection in cats has been shown to have relatively less inflammation than infections in dogs, which may reflect differences in infecting strains and/or underlying unrecognized defects in the immune or inflammatory response.6-8 The cat, in this case, had no significant previous medical history, however, FIV/FeLv status was unknown. In one Australian study, Siamese, Birman and Ragdoll cats were found to be predisposed to Cryptococcus infection.5 This cat was described as a Siamese mix.
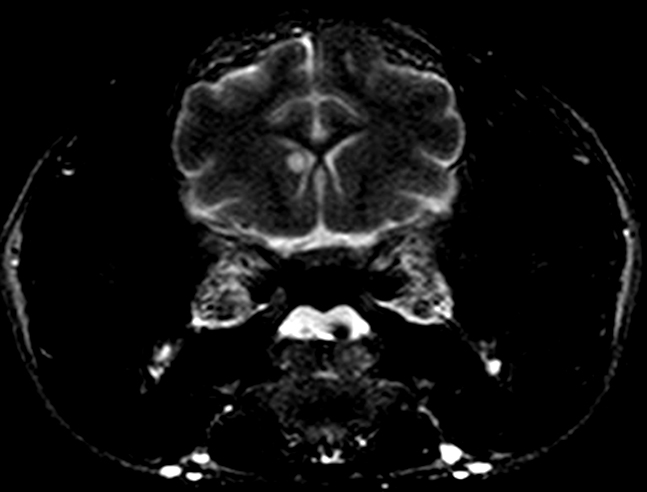
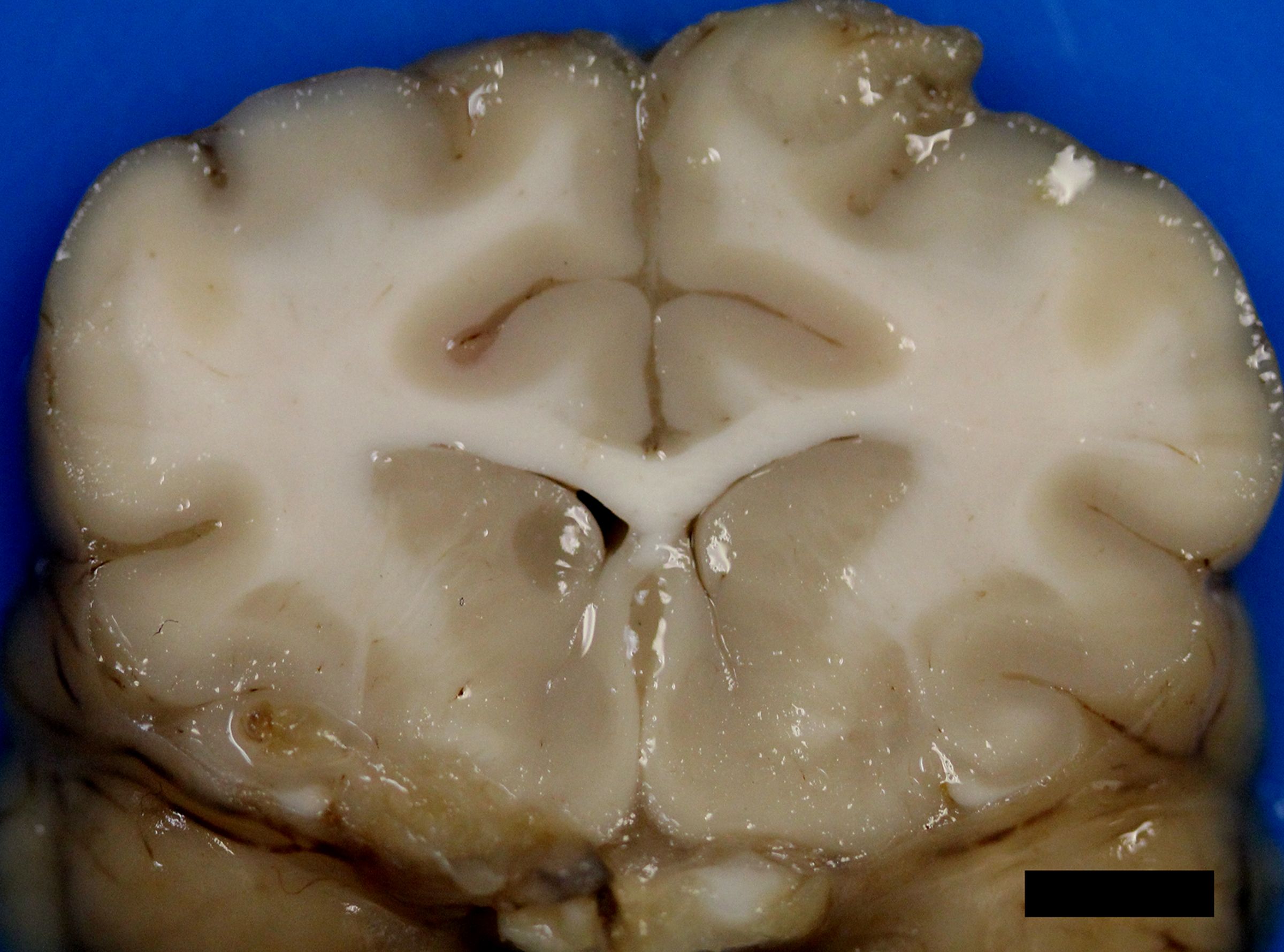
After infection of the respiratory tract, fungal organisms are thought to spread hematogenously via macrophages, frequently to the CNS.5 Extension of nasal cavity infection across the cribriform plate, frontal sinus or along cranial nerves into the brain may also occur.5,7,8 Histologic evaluation of samples from the nasal cavity did not reveal any fungal organisms, and the frontal sinus was grossly normal. Ocular abnormalities can occur in up to one-third of affected cats,5 however, no histologic evidence of infection of the eye or optic nerves was present in this case. Neurologic signs vary depending upon the location of the lesions. Obtundation, behavioral changes, hyperesthesia, tremors, seizures, circling, head pressing, ataxia, paresis, head tilt, vestibular signs, and blindness are common clinical signs.5 The cat, in this case, exhibited obtundation and cluster seizures. Meningeal involvement is common, as in this case, and is considered a predilection site.6 Grossly, the leptomeninges may be unremarkable, or cloudy to thickened, with gelatinous mucoid material and sulcal widening.
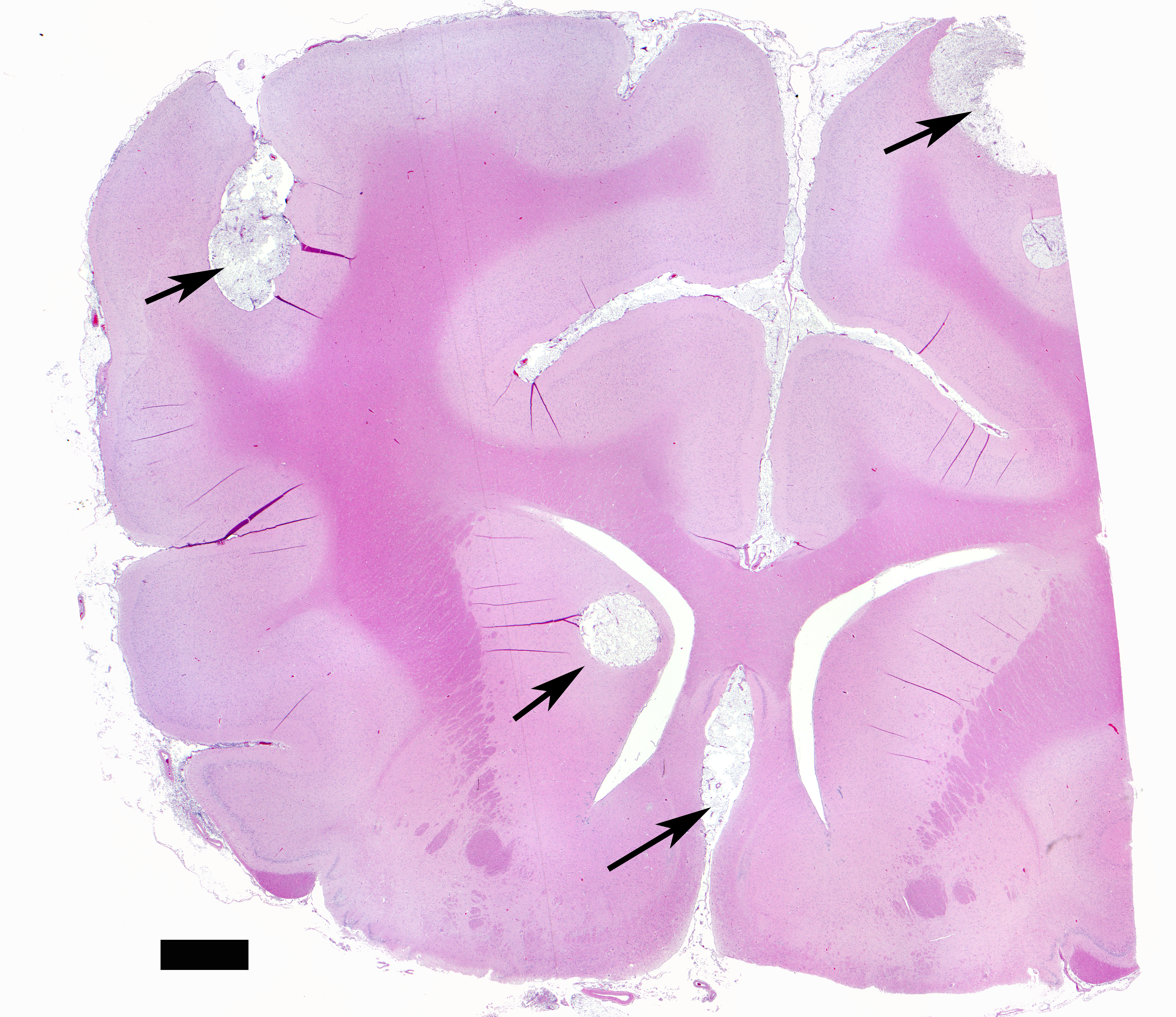
Histologically, tightly packed yeasts give a soap bubble appearance.3,6The leukocytic response can consist of neutrophils, macrophages, multinucleate giant cells, lymphocytes, plasma cells and eosinophils, which is dependent upon host immune status.8 In humans, CNS involvement manifests as meningitis, meningoencephalitis, or Cryptococcomas, which are tumor-like intraparenchymal masses containing yeasts and inflammatory cells,7 similar to the focal nodule observed with imaging and evaluation of the gross specimen in this case. Gelatinous pseudocysts are cystic extensions of VirchowRobin spaces with collections of yeasts, and can also be detected with imaging modalities.7 One study of cats and dogs with CNS Cryptococcus infection identified three histopathologic patterns: 1) Pseudocyst formation with expansion of cryptococcal organisms along Virchow-Robin spaces with multifocal intraparenchymal pseudocysts, 2) Diffuse meningitis only without pseudocysts or parenchymal involvement and 3) meningoencephalitis without pseudocyst formation.7 These patterns were not found to correlate with the type of Cryptococcus sp. or treatment.7
JPC Diagnosis:
Conference Comment:
The contributor noted that the distal aspect of the cerebellar vermis could be observed from the foramen magnum and upon removal of the brain, the vermis was flattened over the brainstem interpreted as cerebellar coning indicating cerebellar herniation. In humans, prognostic indicators for cryptococcosis include abnormal mental status, history of seizures, high antigen titers within serum and cerebral spinal fluid (CSF), poor host inflammatory response, and high CSF pressure.7 This animal likely had increased intracranial pressure secondary to infection leading to herniation of the cerebellum, altered mental state, and seizure activity. Increased intracranial pressure secondary to Cryptococcus sp. infection of the central nervous system is a negative prognostic indicator in humans and animals.3,7
Conference participants discussed different histochemical stains to highlight the thick capsule of this organism. The capsule can by highlighted by mucicarmine and in wet mounts, it stains with India ink. In addition to the periodic acid-Schiff and Grocott's methenamine silver stain run by the contributor, the yeasts also stain with Fontana-Masson stains due to their pro- duction of melanin via a virulence factor, laccase. A positive culture is required for definitive diagnosis.3 Participants also noted the relative lack of inflammation within the neuropil of this animal. As mentioned by the contributor, in contrast to other mycotic infections, such as Blastomyces sp, Coccidioides sp, and Histoplasma sp, the host inflammatory reaction is often quite minimal, likely due the polysaccharide capsular component glucuronoxylomannan, preventing yeast recognition by phagocytes, induction of IL-10, and disruption of dendritic cell activation and maturation.2,5
References:
2. Carroll SF, Guillot L, Qureshi ST. Mammalian model hosts of cryptococcal infection. Comp Med. 2007; 57(1):9-17.
3. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier; 2016:582-583.
4. Lorente-Méndez C, Martínez CM, Corpa JM. Pathology in practice. J Am Vet Med Assoc. 2009; 235:1407- 1409.
5. Malik R, Krockenberger M, OBrien CR, Martin P, Wigney D, Medleau L. Cryptococcosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd ed. St. Louis, MO: Elsevier Inc; 2006:584-598.
6. Summers BA: Inflammatory diseases of the central nervous system. In: Cummings JF, de Lahunta A, eds. Veterinary Neuropathology. St. Louis, MO: Mosby-Year Book, Inc.; 1995:151-155.
7. Sykes JE, Sturges BK, Cannon MS, et al. Clinical signs, imaging features, neuropathology, and outcome in cats and dogs with central nervous system crypto- coccosis from California. J Vet Intern Med. 2010; 24:14271438.
8. Zachary JF: Nervous system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby, Inc.; 2012:891-893.
9. Uchiumi K, Stowe DM, DeVanna JC, Wilcox JL, Neel JA. Pathology in practice. Cryptococcus sp in a dog. J Am Vet Med Assoc. 2014; 245:893-895.