Signalment:
Eight-week-old,
female, Wistar-Han rat, (
Rattus norvegicus).Control rat
from a 7-day exploratory toxicity study.
Gross Description:
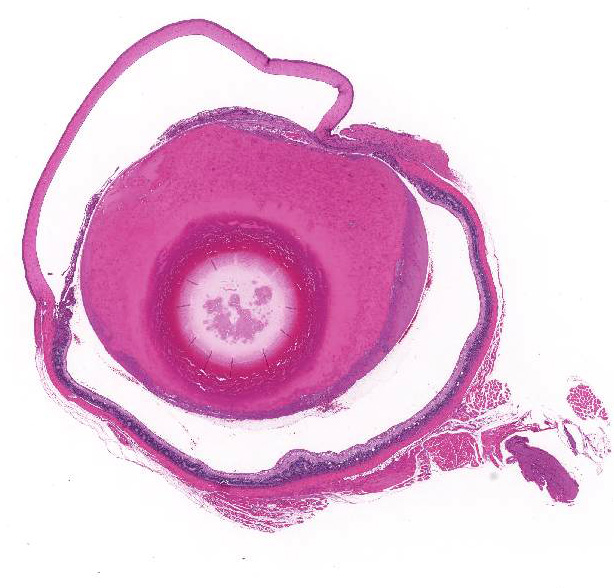
In the right and left eyes, diffusely opaque lens noted at necropsy.
Histopathologic Description:
Eye,
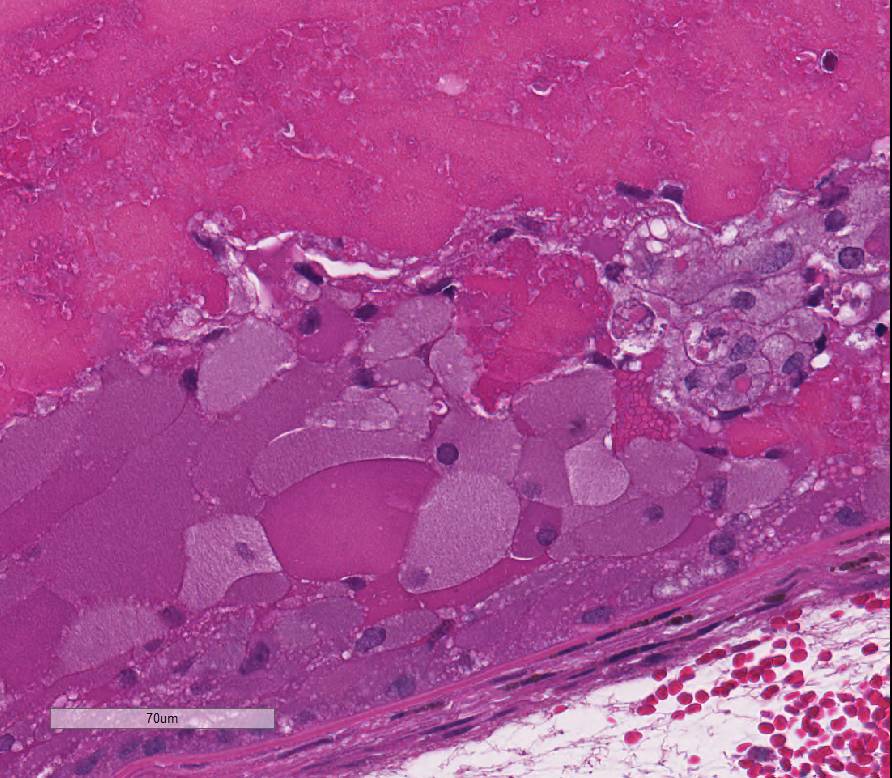
lens: Multifocally, there is disruption and dissolution of the
lenticular fibers often replaced by variably sized, irregular vacuoles which
contain spherical to irregularly-shaped globular eosinophilic aggregates
(Morgagnian globules). Lens epithelial cells are multifocally swollen with
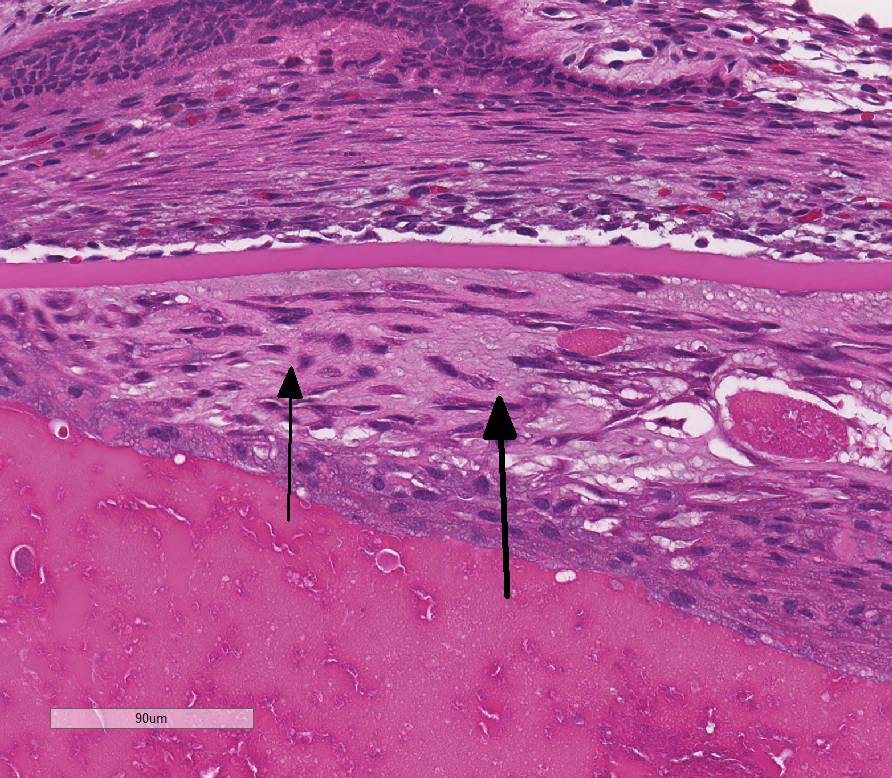
abundant eosinophilic microvacuolated cytoplasm (bladder cells). At one pole, lens epithelial
cells become spindyloid and are separated by fine collagen fibers (fibrous
metaplasia). The
iris is attached to the anterior lens capsule (posterior synechia).
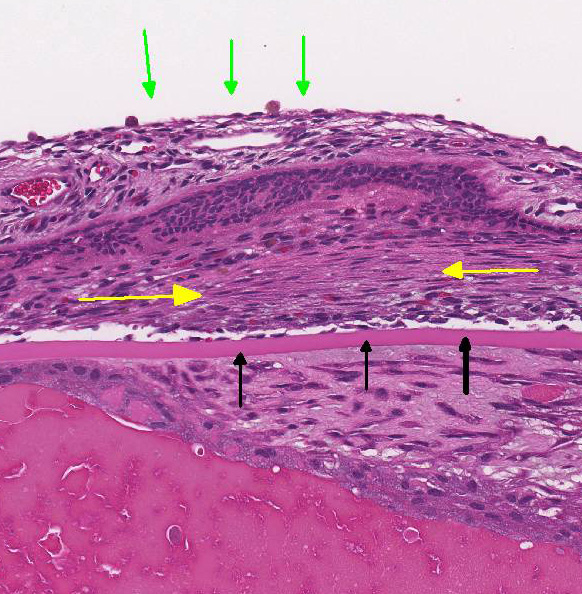
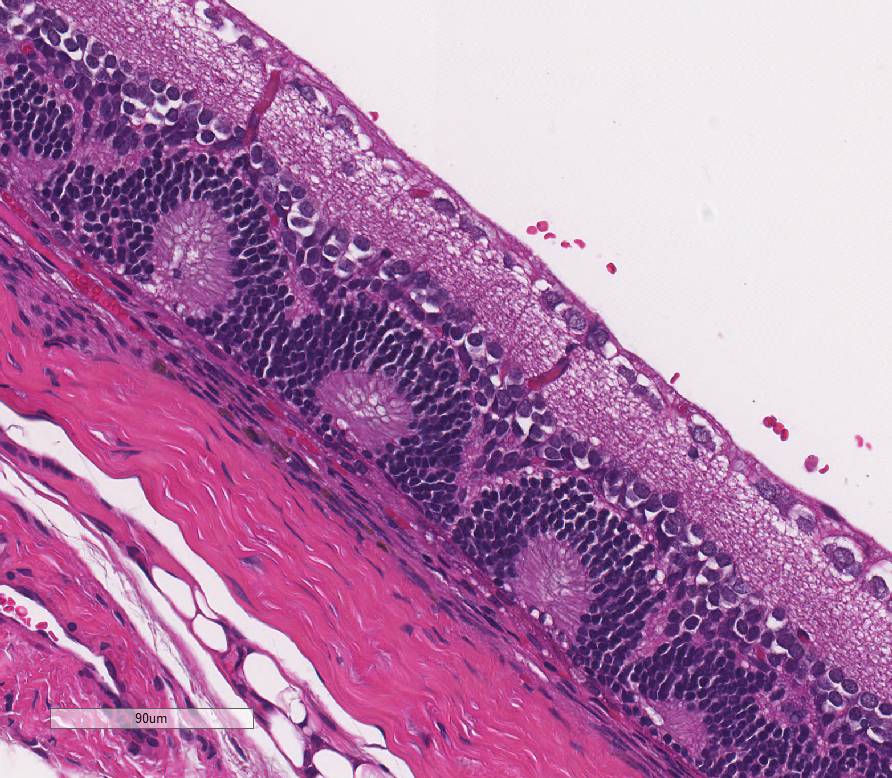
Eye,
retina: Diffusely, the retina is disorganized. The outer nuclear layer forms
numerous rosettes which surround a central space that contain eosinophilic
fibrils (rods and cones). There is thinning of the outer plexiform layer and
multifocal blending of the inner and outer nuclear layers. Retinal pigment
epithelium is frequently vacuolated admixed with occasional nuclear cell debris
and infiltration of macrophages. There is minimal hemorrhage admixed with
scattered macrophages, lymphocytes, and neutrophils within the vitreous space.
Morphologic Diagnosis:
1. Eye, lens: Cataract, diffuse, moderate to severe,
with epithelial hyperplasia, posterior synechiae, and fibrous metaplasia.
2.
Eye, retina: Dysplasia.
3.
Eye, retina: Degeneration, multifocal, mild.
Lab Results:
None.
Condition:
Retinal dysplasia
Contributor Comment:
Cataracts are the most common lenticular disease in aged Sprague-Dawley and
Wistar rats. Cataracts are subclassified by the location in the lens: nuclear
cataract involving the central area of the lens; cortical cataract involving
the lenticular surface; and posterior capsular cataract involving the posterior
surface of the lens and often arising under the capsule.
6 The case
present herein is an example of the latter classification. Posterior capsular
cataracts were described in 32% of aged Wistar rats and overrepresented in
females.
10 The pathogenesis of cataract formation involves initial
lens swelling due to loss of Na-K-dependent ATPase osmotic pumps resulting in
potassium loss and sodium and calcium entry into the lens causing vacuolation
and protein aggregation of the lens epithelium (bladder cell formation) with
subsequent denaturation and hydrolysis of lens fibers (Morgagnian globules).
6
Cataract is considered a major cause of visual impairment in diabetic patients.
The initiating mechanism in diabetic cataract formation is the generation of
polyols from glucose by the aldose reductase pathway, resulting in a similar
increased osmotic stress as described in spontaneous cataract formation leading
to lens fiber swelling and rupture.
6
Retinal
dysplasia is the disorderly proliferation and differentiation of the retina and
is characterized by blending and folding of the retinal layers, rosette
formation, most commonly the inner and outer nuclear layers, and occasionally
degeneration. Retinal dysplasia is an incidental develop-mental anomaly. These
retinal folds and blending of the layers have been described in Wistar rats
occasionally showing micro-phthalmia and cataracts.
6 Retinal
dysplasia is spontaneous or inherited and is rarely progressive.9 A
linear form of retinal dysplasia has been reported in Sprague-Dawley rats at
7-10 weeks of age consisting of loss of the outer layers of the retina
resulting in confluency of the inner nuclear layer with the choroid.9
The findings described in this vehicle-treated animal were considered
incidental.
JPC Diagnosis:
1. Eye,
lens: Cataractous change, subcapsular, diffuse, characterized by Morgagnian
globules, bladder cells, and fibrous metaplasia, with posterior synechia,
Wistar-Han rat, Rattus norvegicus.
2. Eye, retina: Dysplasia.
Conference Comment:
The contributor provides a superb
example of the histologic changes associated with the formation of cataracts
and retinal dysplasia in the rat. Cataracts result from exposure of the lens to
a large variety of insults, including ultraviolet light, physical and chemical
damage, increased intraocular pressure, numerous toxins, direct trauma,
nutrient imbalance, and inflammation.10 Spontaneous cataracts have
also been reported to occur in up to 9.8% of Sprague-Dawley rats and 32% of
aged (>2 years) Wistar rats.2,10 Despite the wide variety of
possible causes, the histologic lesions associated with cataractous change are
relatively stereotypic across species. The lenticular lesions present in this
case include Morgagnian globules, composed of bright eosinophilic globules of
denatured lens protein; bladder cells, which are large foamy nucleated cells
that may represent abortive epithelial attempts at new lens fiber formation;
lens epithelial hyperplasia; and posterior migration of lens epithelium
followed by fibroblastic metaplasia. The latter two changes are associated with
chronic cataract formation.10 Conference participants also noted the
large size of the lens resulting in narrowing of the anterior chamber, which is
a normal finding in the rat.3
This case also generated some spirited discussion
among conference participants regarding whether the retinal changes represent a
dysplastic or degenerative process. The albino Wistar rat is currently one of
the most popular rats used for laboratory research and is exquisitely sensitive
to phototoxicity due to the lack of melanin pigment.3,7 Given the
history of bilateral lesions, strain of the rat in this case, and relatively
young age of the animal, the conference moderator posited that the cataractous
change and retinal lesions could be secondary to phototoxicity. Rats housed in
areas of greater light intensity, such as the outer columns and top racks, are
more susceptible to developing phototoxic lesions.7 Additionally,
light-induced retinal degenerative changes typically manifest as
disorganization and loss of photoreceptor cells in the outer retina,
vacuolation of pigmented retinal epithelium, and accumulation of
intracytoplasmic lipofuscin pigment, all of which are present in this case.1
Conference participants also discussed the
possibility that the lesions in this case represent spontaneous and dysplastic
change. Albino rodents are well known to have several kinds of
spontaneous ocular lesions, including corneal dystrophy (calcium deposition),
cataract, and retinal fold/dysplasia .3 To help elucidate the
possible underlying cause(s) of the retinal changes, this case was studied in consultation with the Dr. Leandro
Teixeira, a board certified veterinary pathologist and recognized expert with
extensive experience in the area of veterinary ocular pathology. Dr. Teixeira
agrees with the contributor that the retinal rosettes, retinal folds, retinal
atrophy, and blending of the inner and outer layers of the retina are common
dysplastic changes in the rat, and are a result of faulty retinal development
rather than a degenerative change. Similar dysplastic lesions can be induced by
the administration of various toxins and carcinogens, such as cytosine
arabinose, cycasin, N-methyl-N-nitrosurea, and trimethylin; however, this
animal is reported to be a control rat and exposure to the aforementioned
compounds is unlikely. Dysplastic lesions can be unilateral or bilateral, as in
this case.9 The lesions in the retinal pigmented epithelium, such as
hypertrophy and vacuolation of pigmented epithelium and accumulation of
lipofuscin, are also common mild cellular degenerative changes secondary to
retinal dysplasia in the rat.
References:
1. Dubielzig RR,
Ketring KL, McLellan GL, Albert DM. The retina. In: Veterinary Ocular
Pathology: A comparative review. St. Louis, MO: Elsevier
Saunders; 2010:360-366.
2. Durand
G, Hubert MF, et al. Spontaneous polar anterior subcapsular lenticular opacity
in Sprague-Dawley rats. Comp Med. 2001; 51:176-179.
3. Kazumoto S,
Tomohiro M, et al. Characteristics of structures and lesions of the eye in
laboratory animals used in toxicity studies. J Toxicol Pathol. 2015;
28(4):181-188.
4. Maggs D, Miller
P, Ron O. Slatters Fundamentals of Veterinary Ophthalmology. 5th
ed. St. Louis, MO: Elsevier Saunders; 2013:452.
5. Mellersh
CS. The genetics of eye disorders in the dog. Canine Genetics and
Epidemiology. 2014:1:3.
6. Pollreisz A and
Schmidt-Erfurth U. Diabetic CataractPathogenesis, Epidemiology and Treatment. J
Ophthalmol. 2010; 1-8.
7. Percy DH,
Barthold SW. Rat. In: Pathology of Laboratory Rodents and Rabbits. 4th
ed. Ames, IA: Blackwell Publishing; 2016:161.
8. Poulsom R and
Hayes B. Congenital retinal folds in Sheffield-Wistar rats. Graefes Arch
Clin Exp Ophthalmol 1988; 226(1):31-3.
9. Schafer KA and
Render JA. Comparative Ocular Anatomy in Commonly Used Laboratory Animals. Eds
Weir AB and Collins W; Springer In: Assessing Ocular Toxicology in
Laboratory Animals. 2012:229.
10. Wegener A,
Kaegler M, Stinn W. Frequency and nature of spontaneous age-related eye lesions
observed in a 2-year inhalation toxicity study in rats.
Ophthalmic Res. 2002; 34(5): 281-7.
11. Wilcock BP, Njaa
BL. Special senses. In: Maxie MG, ed. Jubb, Kennedy and Palmer´s.
Pathology of Domestic Animals. 6th ed Vol. 1. St Louis, MO: Elsevier
Saunders; 2016:436-474.