CASE III: A-808/18 (JPC 4137576)
Signalment:
Muscovy duck (Cairina moschata) flock between 36 and 61 days of age.
History:
Ducks from a meat-producing farm in Delta de l'Ebre, Tarragona (Spain) presented with prostration, diarrhea and high mortality rates. They were previously vaccinated against enteric parvovirus. Ten ducks were submitted for necropsy, histopathology and additional diagnostic tests.
Gross Pathology:
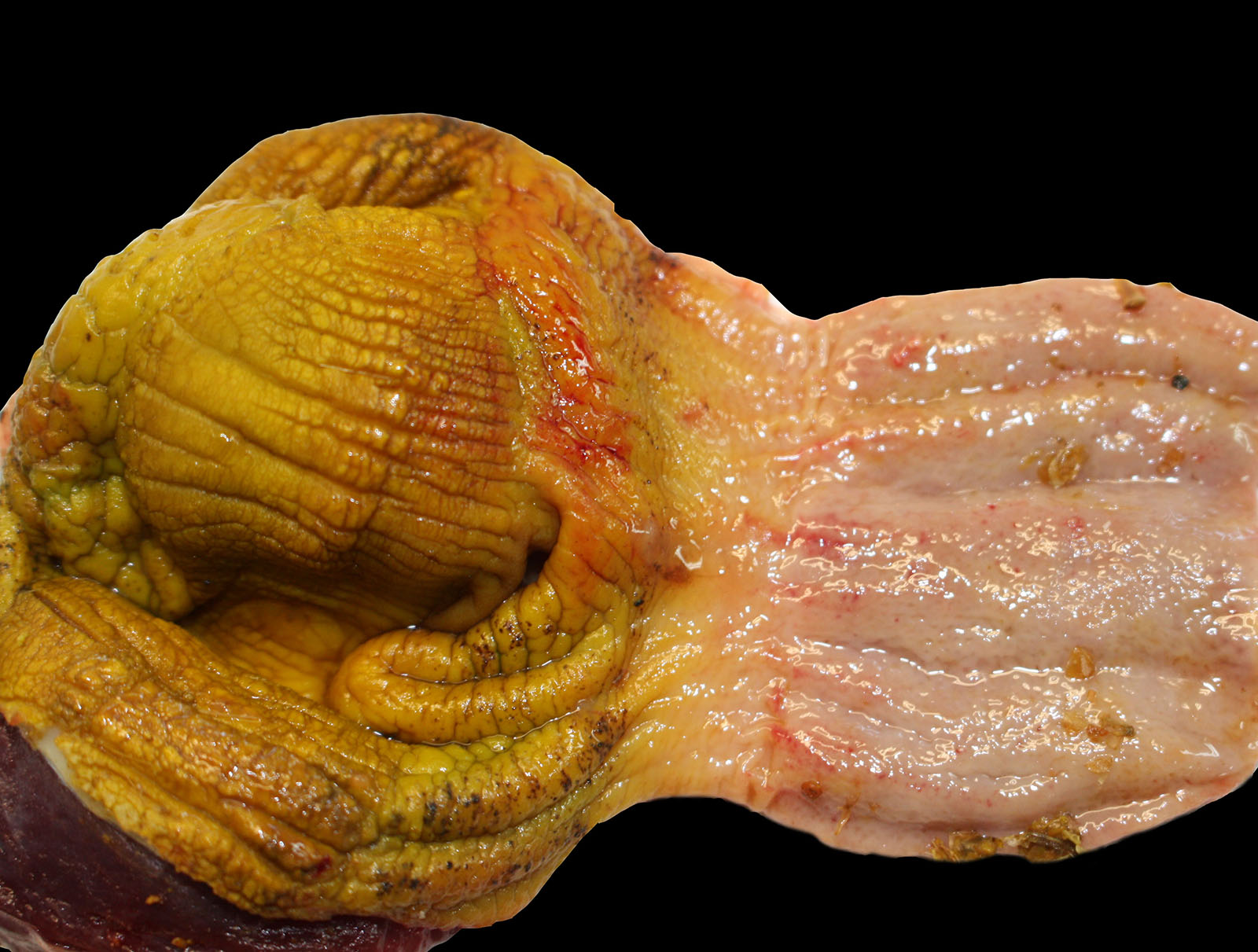
Ten birds were sent for necropsy. Feathers and shanks were spotted with feces (soiled vents). The intestine content was liquid, and the intestinal mucosa showed multifocal petechiae. The intestinal wall was also thickened and multifocally to diffusely reddened, with small amounts of clotted blood.
In some of the birds, liver was enlarged, showing multifocal whitish discolorations. In addition, fibrin deposits were seen over the air sacs and multifocal petechiae in the mucosa of the proventriculus were seen.
In all the birds, cecum scrapings revealed high amounts of flagellated protozoa and helical shaped bacteria.
Laboratory Results:
Cecum samples were taken for microbiologic culture, antibiotic sensitivity test and histopathology. Escherichia coli was isolated in all the cases, being the most abundant growth in 7 out of the 10 submitted birds. In 3 cases, Riemerella anatipestifer was the only and most abundant isolated bacteria.
In addition, tracheal and cloacal swab samples from all birds were taken to rule out Avian Influenza by means of a conventional RT-PCR. In 3 cases, RT-PCR for Riemerella anatipestifer was also performed from heart and liver samples. RT-PCR for Avian Influenza was negative in all the cases. In contrast, RT-PCR for Riemerella anatipestifer was positive in the 3 animals tested.
Microscopic Description:
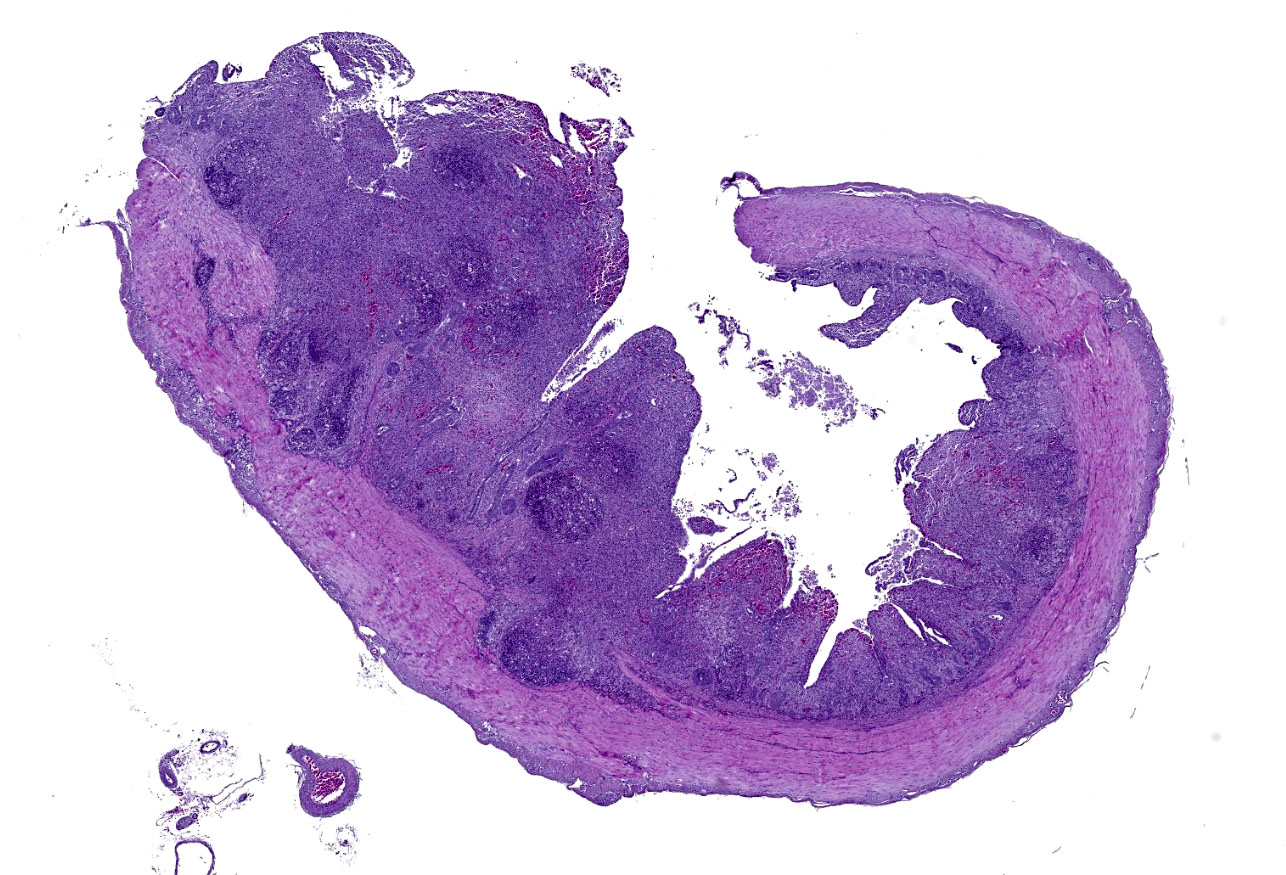
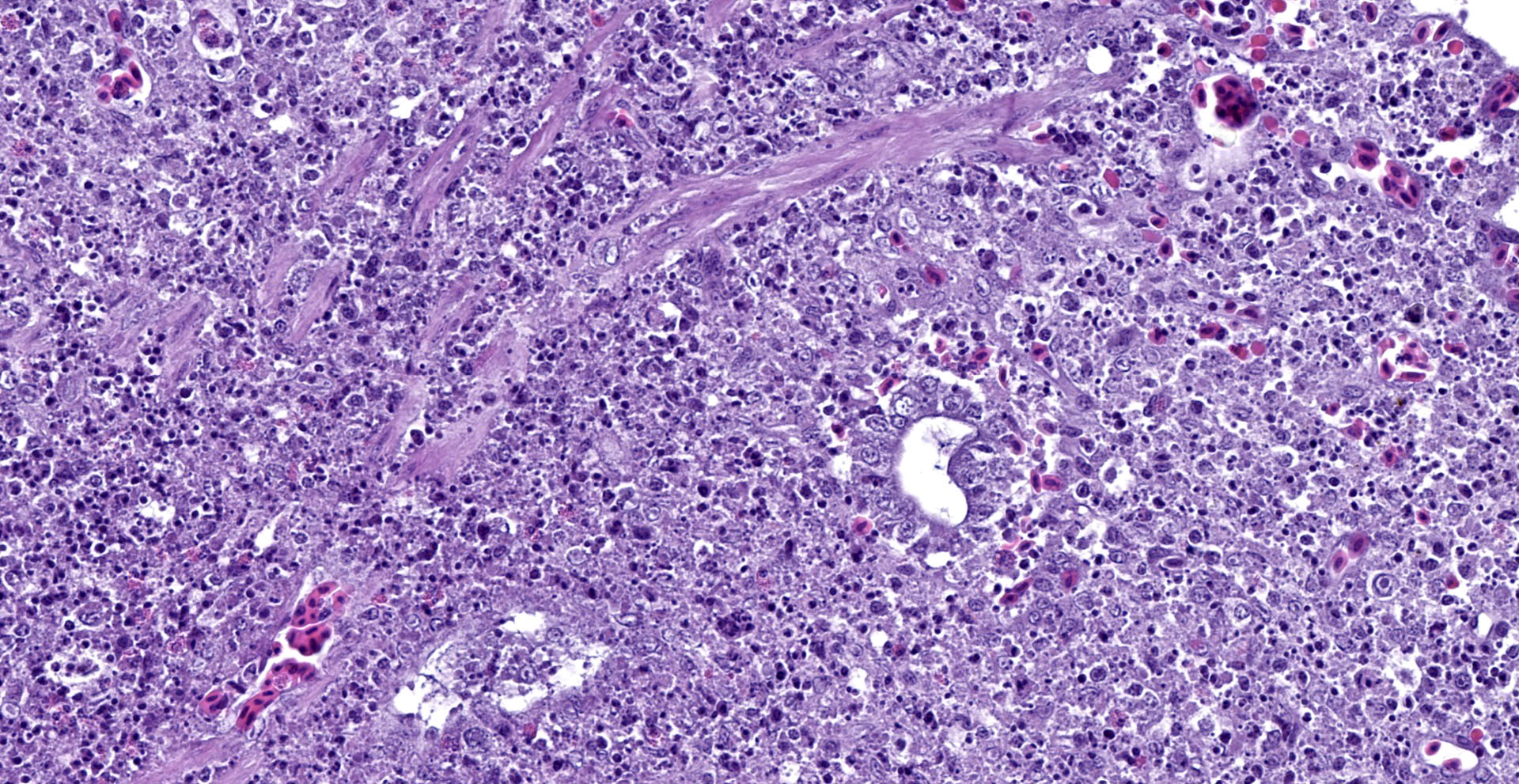
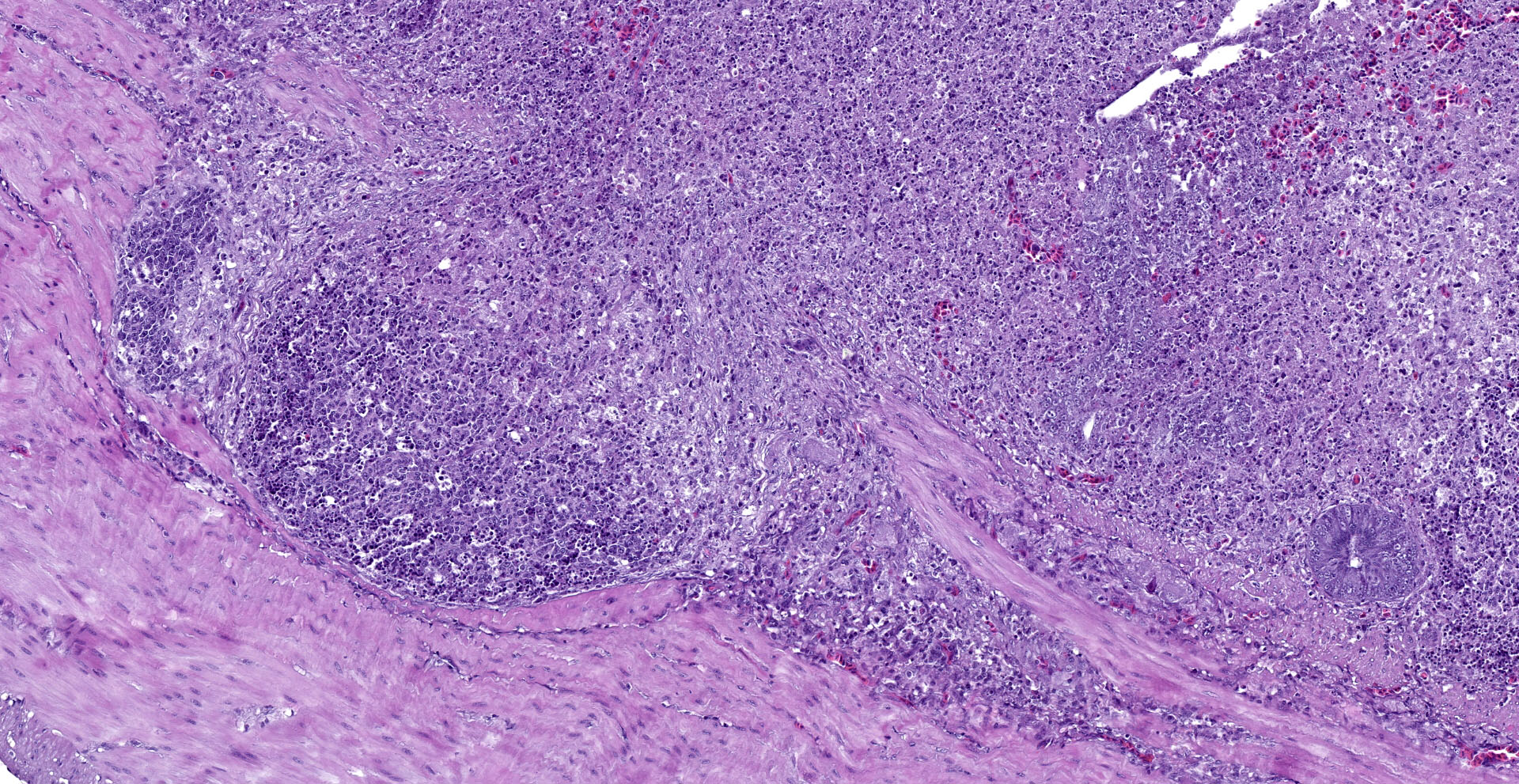
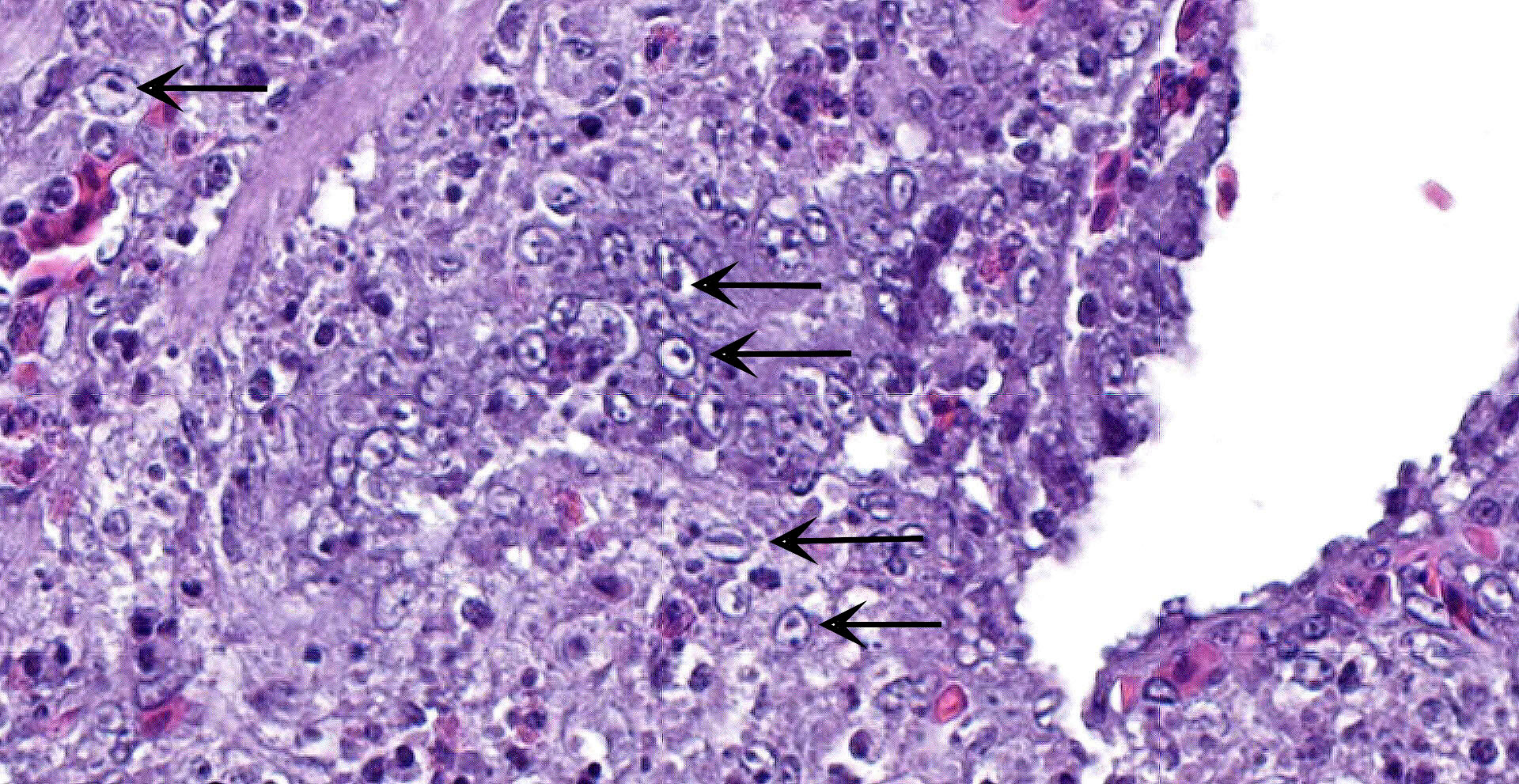
Cecum: There is diffuse loss of the epithelial lining of the mucosa with an increased number of mitotic figures in the Lieberkühn crypts epithelium. The lamina propria show a marked congestion in its upper part and is markedly expanded by high amounts of both, viable and degenerated, heterophils and lymphocytes. Segmental areas of marked distortion of the whole mucosae (epithelium, lamina propria and muscularis mucosae) are seen. These areas are characterized by high amounts of karyolitic, karyorrhectic debris admixed with heterophils and lymphocytes (lytic necrosis) that extend to the underlying lymphoid tissue of the submucosa. Admixed with this necrotic material as well as in Lieberkühn glands some of the degenerated epithelial cells show marginated chromatin and intranuclear and eosinophilic inclusion bodies. In general, submucosal lymphoid tissue is markedly depleted and high amounts of pyknotic, karyolytic and karyorrhectic lymphocytes are seen, together with degenerated heterophils.
Sporadically, either free in the intestinal lumen or multifocally attached to the remnants of the epithelial layer or to the denuded lamina propria, moderate to high amounts of bacillary bacterial colonies are observed. Gram staining revealed they were Gram positive bacilli.
Contributor's Morphologic Diagnoses:
Cecum: subacute, segmental to diffuse necrotizing typhlitis with intranuclear viral inclusion bodies in enterocytes and intralesional bacterial colonies.
Contributor's Comment:
The clinical signs together with both gross and microscopic lesions are suggestive of an infection with Anatid herpesvirus 1 (Genus Mardivirus, family Herpesviridae and subfamily Alphaherpesvirinae), the causative agent of Duck virus enteritis (DVE), also known as Duck Plague. DVE is an acute, contagious disease of ducks, geese and swans of all ages, characterized by vascular damage, tissue hemorrhages, necrosis and depletion of lymphoid organs, and degenerative changes in parenchymatous organs.3 It is known to have a global distribution, wherein migratory waterfowl play a crucial role in disease transmission between continents;1 and it is a cause of significant economic losses in domestic and wild waterfowl due to mortality,
condemnations, and decreased egg production.3
Natural outbreaks of Duck Plague have been reported in birds from 7 days of age to adulthood.1 The incubation period in domestic ducks ranges from 3-7 days. After overt signs appear, death usually follows within 1-5 days.3 DVE can be transmitted by direct contact between infected and susceptible birds or indirectly by contact with a contaminated environment, mainly the water.3 In our case, the duckling farm is set in the biggest delta in Catalonia -Delta de l'Ebre-, where many wild birds inhabit.
Secondary bacterial infections with Pasteurella multocida, Riemerella anatipestifer and Escherichia coli are often seen in natural outbreaks of DVE strains with low virulence in young ducklings as a result of immunosuppressive effect of the virus.3 In this case, Gram positive bacillary bacteria were histologically seen in 4 out of 10 animals, suggesting a secondary infection with Clostridium spp., which can be attributed to a loss of intestinal barrier, dysbiosis and the concurrent immunosuppression by direct effect of DEV.
DVE rapidly spreads with high mortality rates in high densities farms. Breeder ducks are usually maintained at the same location throughout their productive lives; therefore, once a breeder population is exposed to the virus, DVE infection is self-limiting. In contrast, market ducks are progressively moved, as they mature and are relocated in areas formerly occupied by the next oldest age group.3 The latter is the scenario seen in our case, as ducks enter the farm at 1-day old age and are progressively moved to different pens until they go to slaughterhouse.
Regarding latency of the virus, similarly to other herpesviruses, it takes place in the trigeminal ganglion3, although it has been revealed that lymphoid tissues and peripheral blood lymphocytes (PBL) are as well main latency sites for DEV.1 Because of the latency phenomenon, carrier states have been described in wild ducks (especially Mallard ducks, which are considered reservoirs of the virus) and also in recovered birds, that can shed the virus periodically. Posterior reactivation has been blamed for precipitating outbreaks in domestic and migrating waterfowl populations triggered by immunosuppression. Recently it has been reported a reactivation of DEV and subsequent outbreaks due to the stresses resulting from the physiological changes in the duration of daylight and onset of breeding, suggesting a certain seasonality in the course of the disease.1
DVE virus infects and multiplies in mucosal epithelial cells of the gastrointestinal (GI) tract, macrophages and lymphocytes of lymphoid organs (thymus, bursa of Fabricius, spleen), and later in the liver and endothelial cells (particularly those of small blood vessels, like venules and capillaries). Therefore, gross lesions are associated with disseminated intravascular coagulopathy and necrotic degenerative changes in mucosa and submucosa of GI tract in lymphoid and parenchymatous organs. Microscopic lesions initially occur in of blood vessels, especially small blood vessels. The endothelial lining is disrupted and connective tissue of the vessel wall become less compact, leading to hemorrhages. Microscopic changes can be found in any visceral organs including those without gross lesions. A hallmark feature of herpesvirus infections is the presence of eosinophilic intranuclear and less frequently cytoplasmic inclusions, seen in chief sites of viral replication.1,3
Although a presumptive diagnosis can be made on the basis of gross and histopathologic lesions, isolation and identification of DEV confirm the diagnosis. Differential diagnosis for DEV requires consideration of other diseases producing hemorrhagic and necrotic lesions in Anseriformes such as duck virus hepatitis, fowl cholera, necrotic enteritis, coccidiosis, and specific intoxications. Newcastle disease, fowl pox, and fowl plague are reported to produce similar changes in Anseriformes; however, these diseases have been infrequently reported.3
Samples recommended for virus isolation are liver, spleen, bursa, kidneys, PBL and cloacal swabs. Many techniques have been described to reach a certain diagnosis of DVE (virus isolation, propagation and identification; antigen capture ELISA, different types of PCR or loop-mediated isothermal amplification ?LAMP-). The quickest and cheapest tool to use for on-farm and laboratory diagnosis is LAMP.1,3
Because there is no specific treatment for infection with DVE, prevention of the disease is highly recommended. It is achieved by maintaining susceptible birds in environments free from exposure to the virus. These measures include quarantine before introducing new animals into a flock and avoiding direct and indirect contact with possibly contaminated material. After DEV has been introduced, control can be achieved by depopulation, removal of birds from the contaminated environments, sanitation, disinfection, and vaccination of all susceptible ducklings.1,3
Vaccination has been used as a preventive measure and also for controlling disease outbreaks. Inactivated vaccines have been tried but they have not been as efficacious as modified live virus vaccines. Vaccination guideline is as follows: administration by subcutaneous or IM routes in domestic ducklings more than 2 weeks of age. Moreover, flocks maintained for more than a year are revaccinated annually.3
Contributing Institution:
Veterinary Pathology Department,
Veterinary Faculty,
Autonomous University of Barcelona, 08193 Bellaterra, Barcelona, Spain.
JPC Diagnosis:
Cecum: Typhlitis, necrotizing, diffuse, severe, with marked lymphocytolysis and intraepithelial intranuclear viral inclusions.
JPC Comment:
The contributor provides a very thorough synopsis of the epidemiology, pathogenesis, and control measures associated with anatid herpes virus 1, the etiologic agent of duck virus enteritis (DVE), also known as "duck plague". Anatid herpes virus 1 presents a major challenge to the commercial duck industry and is associated with both decreased egg production and increased mortality in unvaccinated flocks, despite availability of effective vaccines.1,2
Baudet first described features consistent with duck plague in 1926 in the Netherlands, detailing an acute hemorrhagic disease amongst domesticated ducks. Baudet concluded the disease was a duck-adapted strain of fowl plague virus as he was able to induce the disease in domestic ducks by infusing filtered liver suspensions but not in chickens. Anatid herpes virus 1 was subsequently isolated and identified in 1949, also in the Netherlands.2 Wild waterfowl were confirmed to be carriers by Friend & Pearson in 1973 following several major outbreaks in domestic and migratory waterfowl. Between 1970-1995, more than 100 outbreaks were reported in regions throughout North America, Europe, the Middle East, and Asia.1 The disease remains to be a significant issue, as demonstrated by a 2016 outbreak in Egypt that affected approximately 1,400,000 unimmunized ducklings across 10 flocks (6 Muscovy and 4 pekin ducks), with mortality rates ranging from 20-60% on affected farms.2
In addition to sudden death, common clinical signs include depression, anorexia, increased thirst, dehydration, weakness, ruffled feathers, photophobia, head and neck tremors, green watery diarrhea, and hematochezia. In addition, penile prolapse is another clinical sign seen in male birds. Death typically occurs within 5 days of the onset of clinical signs in 60-90%, with higher mortality rates affecting breeders than younger birds, likely due to increased stress.1
Interestingly, susceptibility to anatid herpes virus 1 varies within the waterfowl family Anatidae, with some domestic ducks (Aas platyrhynchos) such as the white Pekin and Muscovy ducks (Cairina moschata) being particularly susceptible. Outbreaks have also been reported in non-Anseriformes waterfowl, including common coots (Fulica atra) and crested coots (Flilica cristata). In contrast, mallards (A. platyrhynchos) are resistant to the lethal effects of the virus and are thought to be a natural reservoir. Additional species that have demonstrated a high prevalence of anatid herpes virus 1 via loop-mediated isothermal amplification (LAMP) and also may act as carriers include mute swans (Cygnus olor), greyleg geese (A. anser), tundra bean geese (A. fabalis), and grey herons (Ardea cinerea).1 In addition, the virus is resistant to environmental degradation, retaining its ability to infect new hosts for several weeks in unfavorable environments and is able to survive in a pH range of 4-10. Therefore, commercial operations often enact stringent biosecurity measures, including limiting flock access to areas inhabited by waterfowl, chlorinating water, disinfecting fomites (e.g. footwear), promptly removing carcasses, and quarantining new arrivals, in addition to vaccination programs.1
Ducklings of vaccinated and/or previously challenged breeders possess short lived immunity to anatid herpes virus 1, which can interfere with vaccination efficacy. One study concluded the optimal time for primary vaccination to be at 35 days of age, with a booster 5 months after the initial inoculation.1
References:
1. Dhama K, Kumar N, Saminathan M, et al. Duck virus enteritis (duck plague) - a comprehensive update. Vet Q. 2017;37(1):57-80.
2. El-Tholoth M, Hamed MF, Matter AA, Abou El-Azm KI. Molecular and pathological characterization of duck enteritis virus in Egypt. Transbound Emerg Dis. 2019;66(1):217-224.
3. Metwally SA, Cheng A. Duck virus enteritis (duck plague). In: Swayne DE, ed. Diseases of poultry. 14th ed. Hoboken, NJ: Wiley Blackwell; 2020:460-470.