Signalment:
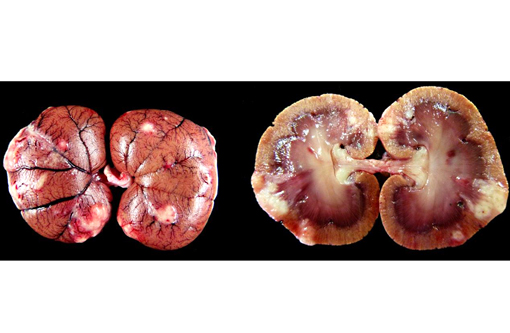
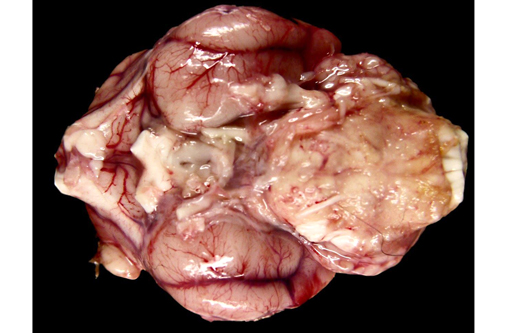
Gross Description:
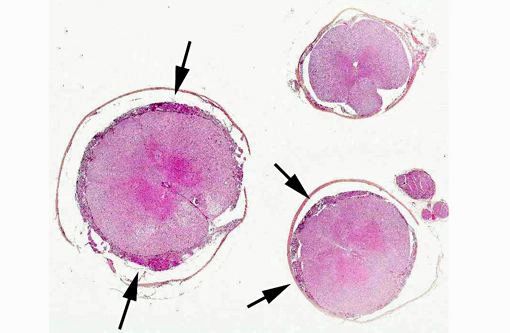
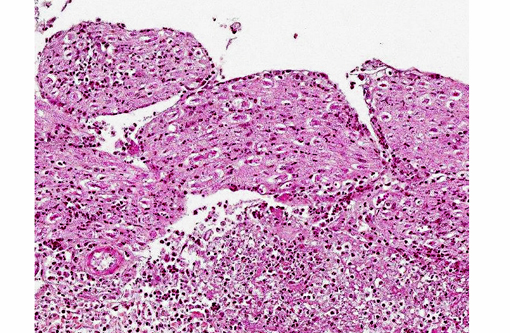
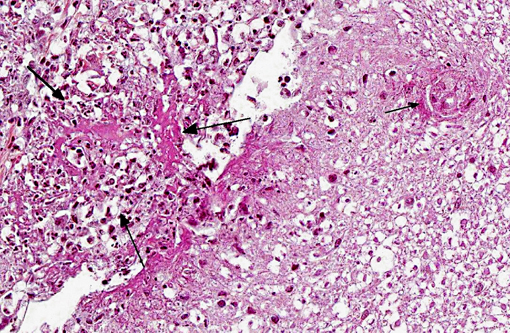
Histopathologic Description:
Morphologic Diagnosis:
1. Meninges: Vasculitis (mainly phlebitis), pyogranulomatous, chronic, diffuse, severe with fibrinoid necrosis and secondary meningitis (due to extension).
2. Spinal cord: Degenerative myelopathy, multifocal, chronic, moderate, with spheroids and dilation of myelin sheaths.
3. Peripheral nerves: Neuritis, pyogranulomatous, chronic, multifocal, severe.
Name of the disease: Feline infectious peritonitis (FIP)
Etiology: Feline infectious peritonitis virus
Lab Results:
| Present case | Reference values | |
| Red blood cell count (/mm3) | 160 | 0 |
| Nucleated cell count (/mm3) | 720 | 2-8 |
| Protein concentration (g/L) | 10.6 | 0-0.3 |
On microscopic examination of the CSF, there was a marked neutrophilic pleocytosis (90%). Some neutrophils were degenerated. Few large and small mononuclear cells were also present (10%). No infectious agents or neoplastic cells were observed.
RT-PCR (reverse transcriptase polymerase chain reaction) analyses for Feline infectious peritonitis Virus (FIPV) were performed on the cerebrospinal fluid and aqueous humor and were strongly positive. RT-PCR analyses on blood and feces were negative.
Condition:
Contributor Comment:
FIP is a fatal immune-mediated disease of cats caused by a member of feline coronaviruses (FCoV), the feline infectious peritonitis Virus (FIPV). Two major forms of FIP have been described: the effusive (wet) form and the non-effusive (dry) form. The distinction between these forms is sometimes arbitrary and they should be regarded as the two extremes of a continuum. Although both forms have been associated with neurological signs, the non-effusive form appears to more commonly produce nervous system lesions.(3,4)
The most widely accepted pathogenesis of FIP considers infection and replication of the virus within macrophages as a key feature. Macrophages can subsequently disseminate the virus through the body (e.g., to liver, visceral peritoneum or pleura, uvea, meninges and ependyma of the brain and spinal cord). In tissues, further replication of the virus results in attraction of neutrophils and macrophages leading to pyogranulomatous inflammation.(10) Both type III and type IV hypersensitivity reactions are believed to occur. The form of FIP is likely determined by the type of immune reaction of the host. Cats that respond with predominantly humoral immunity develop the wet form, whereas those with stronger cell-mediated immunity develop the dry form.(3,13)
The origin of FIPV is still subject to many debates. The most widely accepted hypothesis is that FIPV could be a mutant strain of the feline enteric coronavirus (FECV), another member of the FCoV that causes enteric infection. This mutant strain would be able to infect monocytes and macrophages, causing FIP. FECV is antigenically and morphologically indistinguishable from FIPV. Whether or not FIP develops would also depend on host factors (immune response, cytokine response) in conjunction with viral factors.(5,13)
Feline neurological disease accounts for approximately 10% of total referrals in some feline medicine clinics.(2,8) A specific clinical diagnosis is made in only 30-40% of cases and 30-45% of cases are believed to be infectious in origin. The most common infectious causes of encephalitis in cats are FIP and toxoplasmosis.(8)
Because FIP is a fatal transmissible disease without effective treatment, it is essential for clinicians to make a rapid diagnosis. Histopathology remains the only conclusive means of diagnosis of FIP, particularly in cases of atypical presentation. Surprisingly, FIP has been diagnosed histopathologically in the brains of cats without neurological clinical signs.(4) Occasionally, histopathology can be inconclusive and immunohistochemistry can be useful to confirm or exclude the disease.(7)
In practice, diagnosis is often based on the combination of signalment, history and clinical signs.(8) Although the nervous form of FIP can affect cats at any age, it is considered to be the most common cause of neurological disease in cats less than 4 years of age. Purebred cats are at increased risk and male cats are more frequently affected than females.(4)
Abnormal laboratory findings frequently observed in FIP are hyperproteinemia with a low albumin/globulin ratio (<0.7), anemia, elevated hepatic enzyme levels and hyperbilirubinemia.(17)
CSF analysis is useful in the diagnosis of cats with FIP. Marked elevation of protein concentration (greater than 2 g/L), severe pleocytosis (> 100 cells/μL) with neutrophils (more than 70%) and systemic signs increase the index of suspicion for FIP.(14,16)
Infection by FIPV can be demonstrated by serology and by RT-PCR. Currently available serological tests have low specificity and sensitivity for detection of active infection and cross-react with FECV.(15) RT-PCR is rapid and sensitive but results must be interpreted in the context of clinical findings. RT-PCR on CSF detected only 31% of cats with neurologic FIP. This low sensitivity could be explained by the low CSF cellularity on most cats and the paucity of virus detected immunohistochemically.(6)
JPC Diagnosis:
Conference Comment:
At present, three key features have been identified as prerequisites for the development of FIP lesions: 1) systemic infection with mutated, virulent FCoV (FIPV), 2) effective and sustainable FIPV replication in monocytes, and 3) activation of FIPV-infected monocytes, although the trigger remains unknown. Activated monocytes/macrophages upregulate their expression of adhesion molecules (e.g. CD18), and produce cytokines (such as TNF-α, IL-1b, IL6, G-CSF and GM-CSF), matrix metalloproteinases (e.g., MMP-9), and vascular endothelial growth factor (VEGF). IL-6 stimulates hepatocytes to produce acute phase proteins (such as alpha-1 acid glycoprotein) and drives differentiation of B-lymphocytes into plasma cells. TNF-α, G-CSF and GM-CSF are neutrophil survival factors. IL-1 is a pyrogenic cytokine that activates both B- and T-lymphocytes. MMP-9 is an endopeptidase that breaks down extracellular matrix proteins. VEGF facilitates interaction with activated endothelial cells; it has been proposed that the limited distribution of vascular lesions (only veins in select organs are affected) is a consequence of selective responsiveness of the endothelium.(1,11)
As noted by the contributor, mutated FCoV-infected circulating monocytes are thought to be responsible for viral dissemination, while the nature of the adaptive immune response determines the clinical manifestations of FIP. Cats with a strong cell-mediated immune (CMI) response do not develop FIP, while a weak to nonexistent CMI and strong humoral response results in effusive, or wet, FIP, characterized by vasculitis, peritonitis and intracavitary effusions. In contrast, noneffusive, or dry FIP with widespread pyrogranulomatous inflammation predominates in cats with a moderate CMI response. As the contributor noted, the wet and dry forms of FIP represent a continuum, rather than two specific disease processes; mixed effusive and noneffusive forms are not uncommon.(1,11) Additionally, antibody-dependent enhancement (ADE) can affect the severity of clinical disease. ADE is a phenomenon in which cats with preexisting antibodies (e.g. those that were vaccinated with some trial vaccines or received blood from FCoV positive donors), develop disease more quickly, and perhaps more severely than seronegative cats; however, ADE does not appear to affect seropositive cats infected naturally, who are relatively resistant to reinfection.(1)
FIP has historically been regarded as an immune complex-mediated type III hypersensitivity disease (see WSC 2013-2014, conference 22, case 4), based upon 1) the presence of cell-free fibrinogen, C3, viral antigen, IgG, and complement within leukocytes in vascular and focal granulomatous to necrotizing lesions, 2) FCoV-specific immune complexes in blood and glomeruli and 3) elevated serum γ-globulin and C3 levels. Nonetheless, circulating FCoV-specific immune complexes can also be detected in clinically healthy seropositive cats, and most cases of FIP do not exhibit typical features of immune complex vasculitis, such as the involvement of arteries and the prevalence of neutrophils. So, while type III hypersensitivity may contribute to FIP, it has not yet been confirmed as a crucial pathogenic mechanism. Other authors have proposed that type IV hypersensitivity reactions serve as a driving force behind the granulomatous and necrotizing lesions associated with FIP. As discussed above, activation of viral infected macrophages results in the release of various cytokines and growth factors, specifically VEGF, which is a potent mediator of vascular permeability that may produce vascular leakage and acute phlebitis via alterations in endothelial junctional complexes.(11)
The clinical pathology findings associated with FIP can be somewhat variable and non-specific. When present, effusions are usually classified as a modified transudate, with a high protein content (>3.5 g/dL) and a low nucleated cell count (<5,000 nucleated cells/mL), which parallels a serum hypergammaglobulinemia and decreased albumin:globulin ratio. Serum protein electrophoresis often reveals a polyclonal gammopathy (due to immunoglobulins). Normocytic, normochromic, non-regenerative anemia (or anemia of chronic disease) may also be observed.(1) The release of inflammatory mediators (such as IL-1, IL-6 and TNF-α) results in hepatic production of the acute phase protein hepcidin. Hepcidin induces the degradation of the iron export protein ferroportin, effectively sequestering iron within macrophages and enterocytes and ultimately resulting in decreased iron availability with subsequent anemia.(9) As noted by the contributor, CSF evaluation can also be useful in reaching a diagnosis of FIP; however, obtaining a specimen is often quite difficult due to the high viscosity of fluid from increased protein levels.(1) Serum levels of the acute phase protein, α1-acid glycoprotein (AGP) are often significantly elevated (>3 mg/ml), but this is not specific for FIP and may occur with other inflammatory conditions, neoplastic diseases (lymphoma) or in asymptomatic FCoV carriers, especially from households with endemic infection.(11)
Table 1. Select coronaviruses of veterinary importance.12,18
| Group 1a | Disease/Symptoms | |
| -Ç-ó Feline enteric coronavirus -Ç-ó Mutated feline enteric coronavirus (FIP) | -Ç-ó None/mild gastroenteritis -Ç-ó Peritonitis, vasculitis, granulomatous inflammation |
|
| Canine coronavirus | Mild gastroenteritis | |
| Transmissible gastroenteritis virus of swine (TGE) | Gastroenteritis, watery diarrhea, dehydration | |
| Porcine respiratory coronavirus (PRCV) | Mild respiratory disease/interstitial pneumonia | |
| Ferret (FRECV) & mink enteric coronaviruses | Epizootic catarrhal enteritis | |
| Ferret systemic coronavirus (FRSCV) | FIP-like (dry/granulomatous form) systemic dz | |
| Group 1b | ||
| Porcine epidemic diarrhea virus | Gastroenteritis, watery diarrhea, dehydration | |
| Group 2a | ||
| Porcine hemagglutinating encephalomyelitis virus | Encephalomyelitis, wasting, muscle tremors | |
| Mouse hepatitis virus | Hepatitis, enteritis, nephritis, demyelinating encephalomyelitis | |
| Sialodacryoadenitis virus of rats | Salivary/lacrimal gland necrosis/inflammation, chromodacryorrhea | |
| Bovine coronavirus | Gastroenteritis (winter dysentery), respiratory disease | |
| Group 2b | ||
| Severe acute respiratory syndrome (SARS) coronavirus | Humans | |
| SARS coronavirus | Civets, cats, bats; subclinical | |
| Group 3 | ||
| Avian infectious bronchitis virus | Tracheobronchitis, nephritis, rales | |
| Turkey coronavirus/Bluecomb virus | Enteritis, cyanosis |
References:
1. Addie DD, Jarrett O. Feline coronavirus infections. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd ed. St. Louis, MO: Saunders; 2006:91-100.
2. Bradshaw JM, Pearson GR, Gruffydd-Jones TJ. A retrospective study of 286 cases of neurological disorders of the cat. J Comp Path. 2004;131:112120.
3. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 2. 5th ed. London, UK: Saunders Elsevier; 2007:290-293.
4. Diaz JV, Poma R. Diagnosis and clinical signs of feline infectious peritonitis in the central nervous system. Can Vet J. 2009;50(10): 10911093.
5. Foley JE, Leutenegger C. A review of coronavirus infection in the central nervous system of cats and mice. J Vet Intern Med. 2001;15(5): 438444.
6. Foley JE, Lapointe JM, Koblik P, Poland A, Pedersen NC. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med. 1998;12(6): 415423.
7. Giori L, Giordano A, Giudice C, Grieco V, Paltrinieri S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J Small Anim Pract. 2011;52:152-157.
8. Gunn-Moore DA, Reed N. CNS disease in the cat: current knowledge of infectious causes. J Fel Med Surg. 2011;13(11): 824836.
9. Keel SB, Abkowitz JL. The microcytic red cell and the anemia of inflammation. N Engl J Med; 2009;361(19): 1904-1906.
10. Kent M. The cat with neurological manifestations of systemic disease. Key conditions impacting on the CNS. J Fel Med Surg. 2009;11(5): 395407.
11. Kipar A, Meli ML. Review Feline infectious peritonitis: Still an Enigma? Vet Pathol. 2014:51(2): 505-526.
12. MacLachlan NJ, Dubovi EJ. Fenners Veterinary Virology. 4th ed. London, UK: Academic Press; 2011:393-412.
13. Myrrha LW, Miquelitto Figueira Silva F, Fernandes de Oliveira Peternelli E, Silva Junior A, Resende M, Rog+â-¬ria de Almeida M. The paradox of Feline Coronavirus pathogenesis: a review. Adv Virol. 2011;2011:1-8.
14. Rand JS, Parent J, Percy D, Jacobs R. Clinical, cerebrospinal fluid, and histological data from twenty-seven cats with primary inflammatory disease of the central nervous system. Can Vet J. 1994;35(2): 103110.
15. Sharif S, Suri Arshad S, Hair-Bejo M, Rahman Omar A, Allaudin Zeenathul N, Alazawy A. Diagnostic methods for Feline Coronavirus: a review. Vet Med Int. 2010;2010:1-7.
16. Singh M, Foster DJ, Child G, Lamb WA. Inflammatory cerebrospinal fluid analysis in cats: clinical diagnosis and outcome. J Fel Med Surg. 2005;7(2): 7793.
17. Tsai HY, Ling-Ling C, Chao-Nan L, Bi-Ling S. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in Taiwan. J Feline Med Surg. 2010;13:74-80.
18. Wise AG, Kiupel M, Maes RK. Molecular characterization of a novel coronavirus associated with epizootic catarrhal enteritis (ECE) in ferrets. Virology. 2006;349:164174.