Signalment:
Adult, male, Fischer 344 rat (Rattus norvegicus).
This rat was euthanized after presenting for rolling and inability to maintain balance.
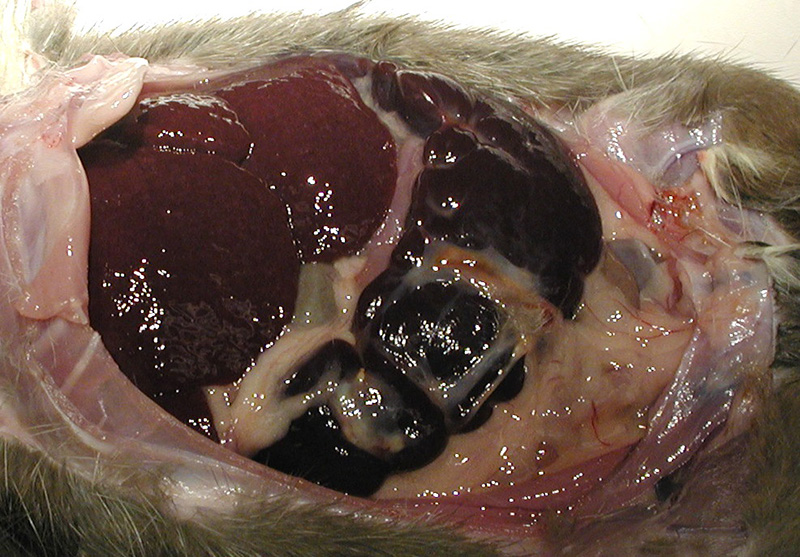
Gross Description:
The spleen was dark red, markedly enlarged (9 x 3 x 1.5 cm) and occupied 2/3 of the abdominal cavity, compressing adjacent organs. The surface was irregular and traversed by prominent white-tan fibrous adhesions. The liver was dark red and moderately enlarged.
Histopathologic Description:
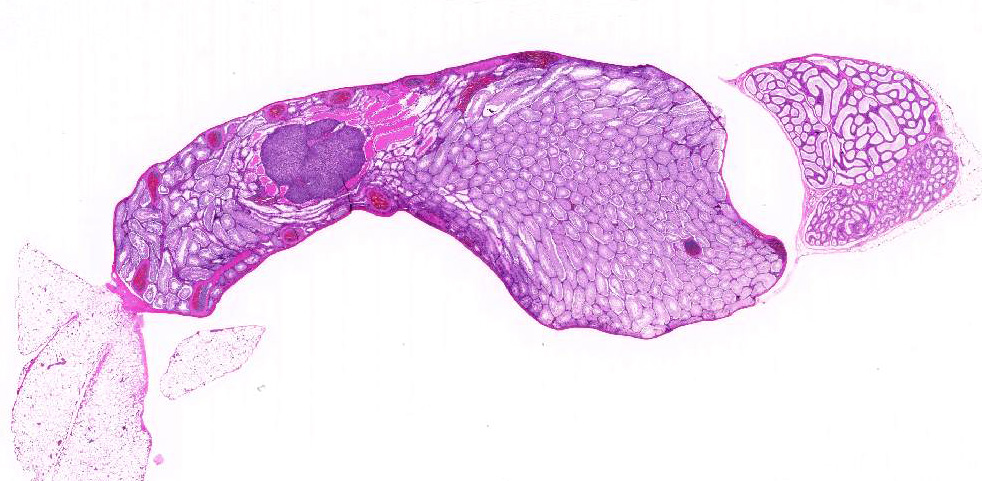
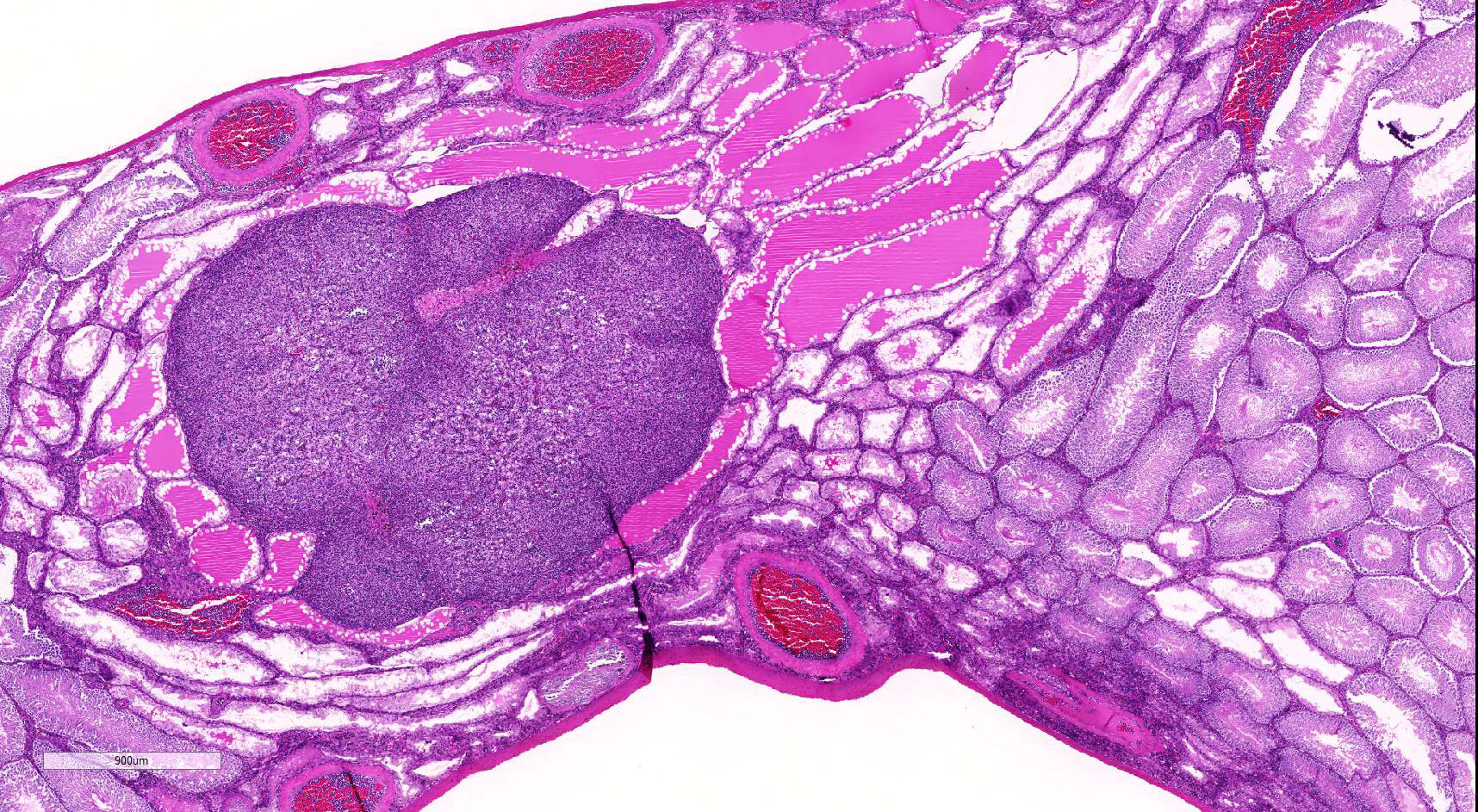
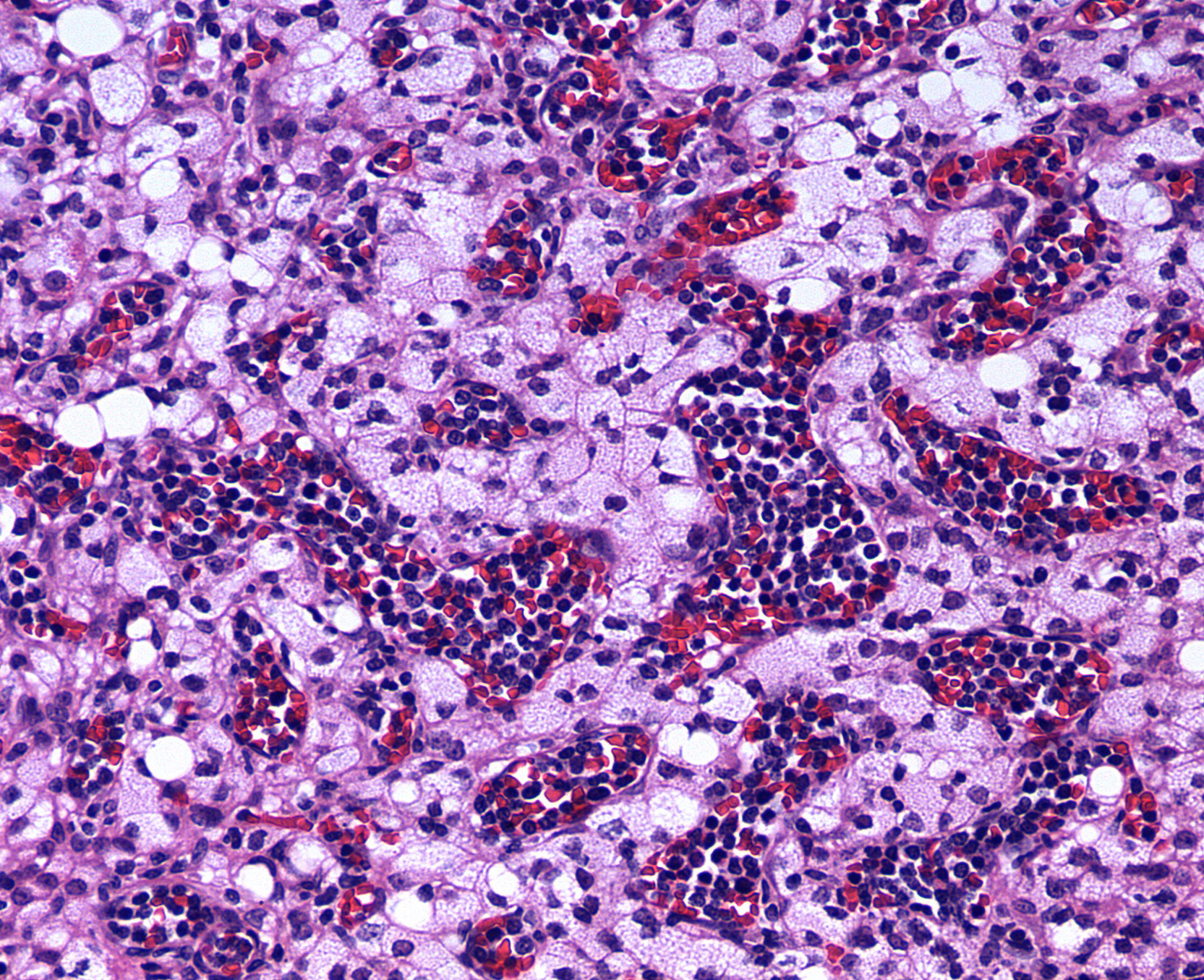
Testis: Testicular blood vessels are filled with neoplastic round cells resembling ly-mphocytes, with irregularly round, 6-10 micron diameter hyperchromatic nuclei and scant pale eosinophilic cytoplasm frequently forming thin rims around the nuclei. Mitotic figures are rare. Replacing the testicular parenchyma is another neoplasm, 3 x 4 mm, well demarcated, unencapsulated and multilobular, composed of sheets and packets of round to polygonal cells supported by fine fibrovascular stroma. The neoplastic cells have variably distinct cell borders, abundant eosinophilic finely vacuolated cytoplasm, and oval, central nuclei with finely stippled chromatin and a single, variably distinct basophilic nucleolus. Mitotic figures are rare. The adjacent seminiferous tubules are compressed and shrunken, lined only by Sertoli cells with an absence of germ cells.Some contain rounded germ cells with eosinophilic cytoplasm and nuclear condensation or multinucleated spermatid giant cells. Others are dilated and contain variable amounts of homogeneous eosinophilic material.
Morphologic Diagnosis:
1. Testis, vessels: Lymphocytic leukemia (Large Granular Lymphocyte Leukemia)
2. Testis: Interstitial cell tumor
3. Testis, seminiferous tubules: Tubular degeneration and atrophy, multifocal, moderate
Lab Results:
None reported.
Condition:
Contributor Comment:
Large granular lymphocyte leukemia and interstitial cell tumors are two of the most common neoplasms in aged Fischer 344 (F344) rats. Large granular lymphocyte (LGL) leukemia, also known as mononuclear cell leukemia or Fischer rat leukemia occurs commonly in the F344 rat but is also reported in other strains. It is a rapidly fatal, age-related neoplasia, with a significantly increased risk after 20 months of age.4 Clinically, rats can exhibit inactivity, weight loss, pale eyes, icterus, and palpable splenomegaly.6
Common clinical pathological abnormalities include hemolytic anemia and th-rombocytopenia. Gross lesions include a ch-aracteristically markedly enlarged, often friable spleen and an enlarged, mottled liver. Enlarged mesenteric lymph nodes and hemorrhage in the lungs, lymph nodes and brain can also be present.4
Histologically, LGL leukemia most con-sistently demonstrates diffuse leukemic infiltration of the red pulp, where the neoplasm is believed to arise, with variable lymphoid depletion.4,5 -The liver and spleen are generally always infiltrated by neoplastic LGLs. In advanced stages, a general leukemia is present, and vessels in lymph nodes, lung, and brain are often infiltrated by neoplastic cells. Bone marrow involvement, if present, occurs late relative to involvement of the spleen.4 -Rats with LGL leukemia can also have focal accumulations of neoplastic lymphocytes,7 such as were observed in the brain in this case, which led to the neurological clinical signs.
Large granular leukocytes have distinctive azurophilic cytoplasmic granules in blood smears; granules are generally not discernible on histology sections.5 Well-differentiated LGLs may have reniform nuclei and cytoplasmic granules; poorly differentiated LGLs have few or inapparent granules.6 LGL cells are generally pleomorphic and 10-15 microns in diameter. Many studies have demonstrated natural killer cell activity of LGLs, but the exact cell of origin is still not fully understood.6,8
Interstitial, or Leydig, cell tumors are the most common testicular neoplasms in rats, dogs, cats and bulls.1,2 Fischer 344 rats have an incidence approaching 100% by 24 months of age.1 Grossly, interstitial cell tumors tend to be well-circumscribed, tan to yellow-orange, soft masses. There can be multiple tumors and they can be unilateral or bilateral. Histologically, interstitial cell tumors are unencapsulated and composed of uniform round or polyhedral neoplastic cells with abundant eosinophilic finely granular or vacuolated cytoplasm. Peripheral sem-iniferous tubules are often compressed and show variable degrees of degeneration and atrophy. Nuclei are generally round, centrally-placed and have single nucleoli. Mitotic rates are generally low. Interstitial cell hyperplastic lesions, probably pre-neoplastic lesions, can be more diffuse, occur between tubules rather than replace them, and have a diameter smaller than three seminiferous tubules.1 A much less common and more pleomorphic proliferative in-terstitial cell lesion with cellular atypia and invasion of adjacent tissue, including vessels or the capsule, or with metastases warrants the diagnosis of interstitial cell carcinoma.1
JPC Diagnosis:
1. Testis, blood vessels:- Mononuclear cell leukemia.
2. Testis: Leydig cell adenoma.
3. Testis, seminiferous tubules: De-generation, atrophy and loss with marked aspermatogenesis and spermatid giant cells.
Conference Comment:
Conference participants discussed the nomenclature of large granular lymphocytic (LGL) leukemia. We prefer the nomenclature noted above, in agreement with INHAND Project (In-ternational Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice). The granular descriptor refers to the cytologic appearance of neoplastic cells when stained with a Wright-Giemsa stain; the granules cannot be visualized in histologic sections stained with hematoxylin and eosin.The moderator also mentioned that in some severe cases, extravascular infiltrates of neoplastic cells can be seen as well as circulating cells with mitotic figures. The neoplasm is a heterogeneous, non-B, non-T cell leukemia with neoplastic cells having natural killer cell activity; cells are negative for the immunohistochemical stain leukocyte common antigen (LCA, CD45).Icterus is often seen in affected animals se-condary to hemolytic anemia; the leukemia cells will also phagocytose erythrocytes and their granules can be erythrolytic.Due to the high incidence of this condition in Fischer 344 rats, Sprague-Dawley and Wistar rats (as opposed to Fischer 344 rats) are used more commonly in toxicology studies.
Early diagnosis of LGL leukemia can be challenging, but the presence of leukemia cells in hepatic sinusoids may aid in the diagnosis.Other hepatic changes include nodular regenerative hyperplasia, ch-aracterized by centrilobular atrophy and periportal hypertrophy of hepatocytes secondary to anemia-related ischemic hepatocellular injury; endothelial injury may also play a role in some cases. Splenic congestion, a common and consistent finding, and is postulated to be related to portal hypertension in addition to proliferation of neoplastic LGL leukemia cells in the spleen.The precise cause or pathogenesis of portal hypertension in this condition is unclear.3
As noted in the contributors comments, Leydig cell hyperplasia is a differential diagnosis for Leydig cell tumor.Neoplastic lesions are considered to be a continuum or progression from hyperplastic lesions in most cases.Unlike hyperplasia, however, Leydig cell tumors will generally de-monstrate compression of adjacent testicular parenchyma and neoplastic cells will be larger, with larger nuclei and may (or may not) demonstrate atypia. Hyperplastic lesions may be focal, multifocal or diffuse, with diffuse being most common, and cells may demonstrate prominent vacuolation;however, hyperplastic lesions will not compress the surrounding tissue. Another criterion which may aid in the diagnosis of less straightforward cases is the designation of a proliferative Leydig cell lesion with a diameter of greater than three seminiferous tubules as a neoplasm (Leydig cell adenoma).1
The pathogenesis of Leydig cell hyperplasia is related to elevated luteinizing hormone levels, paracrine stimulatory factors within the testis or can be secondary to decreased spermatogenesis.1 Restriction of dietary intake may decrease the incidence of interstitial cell tumor but is not documented to decrease the incidence of LGL leukemia.7 A diffuse distribution of hyperplastic lesions is more common in the mouse as well as in the rat.
References:
1. Creasy D, Bube A, de Rijk E, et al. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system.Toxicol Pathol. 2012; 40(6):40S-121S.
2. Ladds, PW. The male genital system.In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals. 4th ed. London, UK: Academic Press; 1993:471-529.
3. Shiga A, Narama I.Hepatic lesions caused by large granular lymphocyte leukemia in Fischer 344 Rats: Similar morphologic features and morphogenesis to those of nodular regenerative hyperplasia (NRH) in the human liver. Toxicol Pathol. 2015; 43(6):852-864.
4. Stromberg PC and Vogtsberger LM. Pathology of the mononuclear cell leukemia of Fischer rats. I. Morphologic studies. Vet Pathol. 1983; 20:698-708.
5. Suttie, AW. Histopathology of the spleen. Toxicol Pathol. 2006; 34(5):466-503.
6. Thomas J, Haseman JK, Goodman JI, et al. A review of large granular lymphocytic leukemia in Fischer 344 rats as an initial step toward evaluating the implication of the endpoint to human cancer risk assessment. Toxicol Sci. 2007; 99(1):3-19.
7. Thurman JD, Bucci TJ, Hart RW, et al. Survival, body Weight, and spontaneous neoplasms in ad libitum- fed and food-restricted Fischer-344 rats. Toxicol Pathol. 1994; 22(1):1-9.
8. Ward, J. M., and Reynolds, C.W. (1983). Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F344 rats. Am J Pathol. 1983:111(1):110.