Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 17
January 31st, 2018
CASE IV: 2017 B (JPC 4101690).
Signalment: 3-year-8-month-old, female, Holstein, Bos taurus, bovine.
History: An idiopathic disease occurred in 4 of 80 dairy cows during the month of April. The affected cows showed anorexia, and dystaxia and/or astasia. Three of the affected cows were slaughtered; a fourth was submitted for necropsy. Clinically, this cow had sudden astasia 5 days before necropsy, and became hyposensitive. The cow stretched its forelimbs frequently and often had spasms in the left hindlimb.
Gross Pathology: Mild congestion was observed in the brain. Muscles in the left hindlimb, in which spasms had been observed, were dark-red.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): None provided.
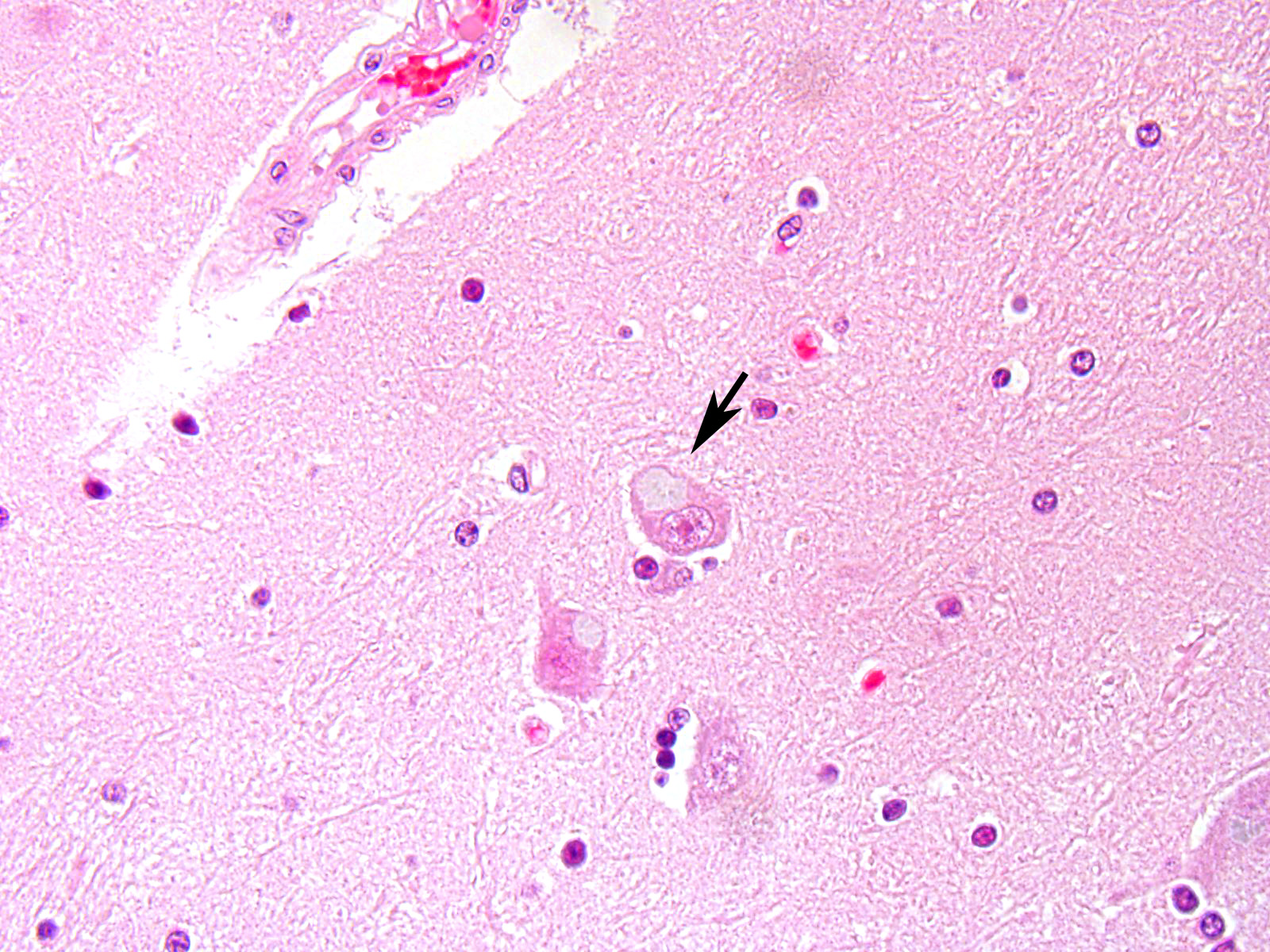
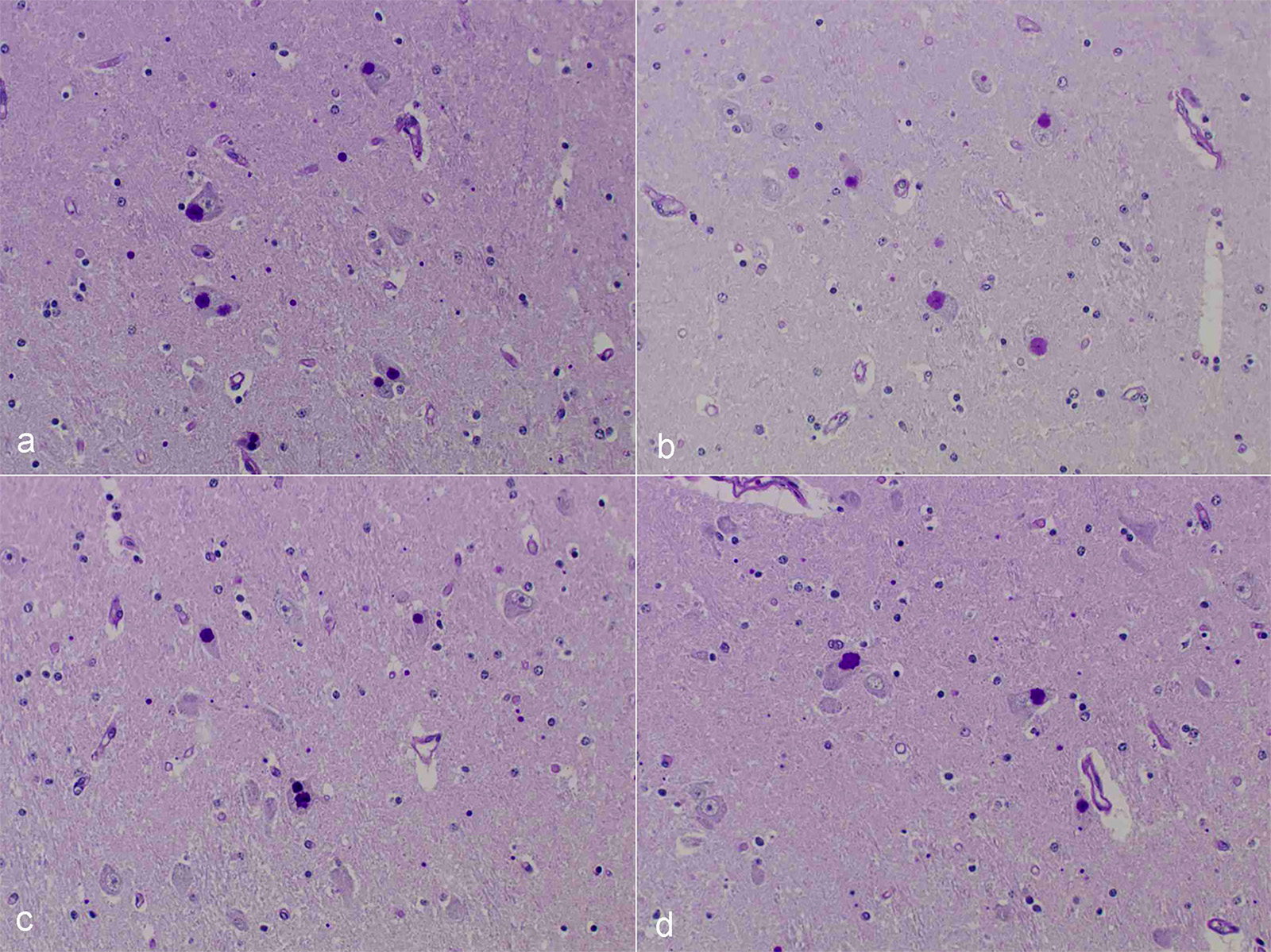
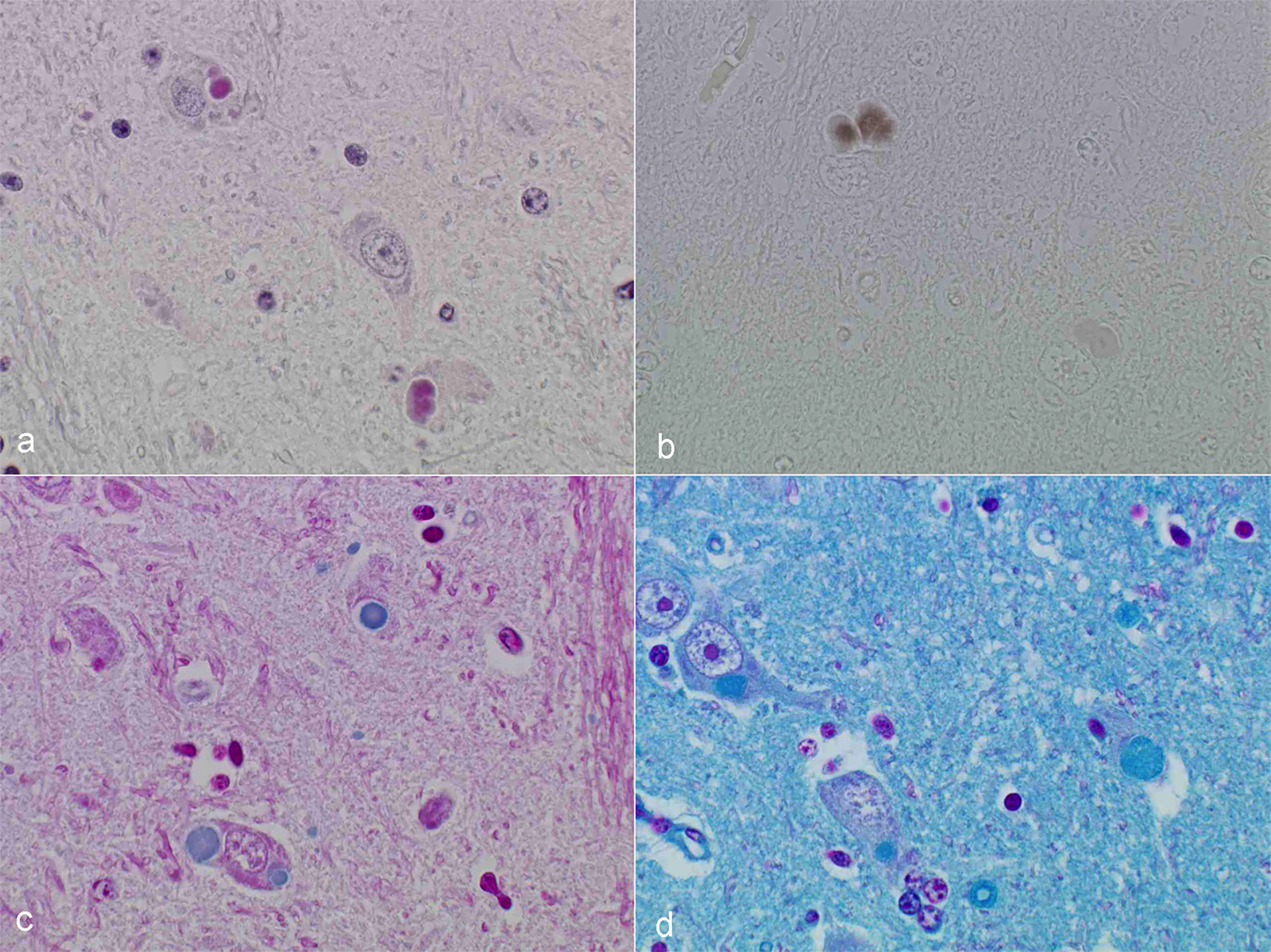
Microscopic Description: After hematoxylin and eosin (HE) staining, numerous basophilic inclusion bodies approximately 15 µm in diameter were observed in the perikarya and neuropil. The inclusion bodies in the perikarya were round to cauliflower-shaped, and some had dense cores. Bodies in the neuropil were round and occasionally concentric, and stained homogenously. They were positive on PAS stain and resistant to diastase and β-amylase digestion, but partly sensitive to α-amylase digestion. Further, these bodies stained red with Best’s carmine, brown with iodine, clear blue with Alcian blue pH2.5 and blue with colloidal iron. The bodies were mostly negative on Nissl stain, but the cores of some bodies were positive.
The inclusion bodies in perikarya were numerous in the diencephalons, especially in the dorsal region of the thalamus. In neuropil, the bodies were observed throughout the central nervous system (CNS), but few were observed in the cerebellum or spinal cords. Such lesions were seen only in the CNS.
Immunohistochemical examination was performed using anti-ubiquitin polyclonal antibodies and anti-GFAP antibodies. The inclusion bodies were slightly reactive with anti-ubiquitin antibodies. In sections with anti-GFAP antibodies, the inclusion bodies were not surrounded by GFAP-positive cells, indicating they rarely occur in astrocytes.
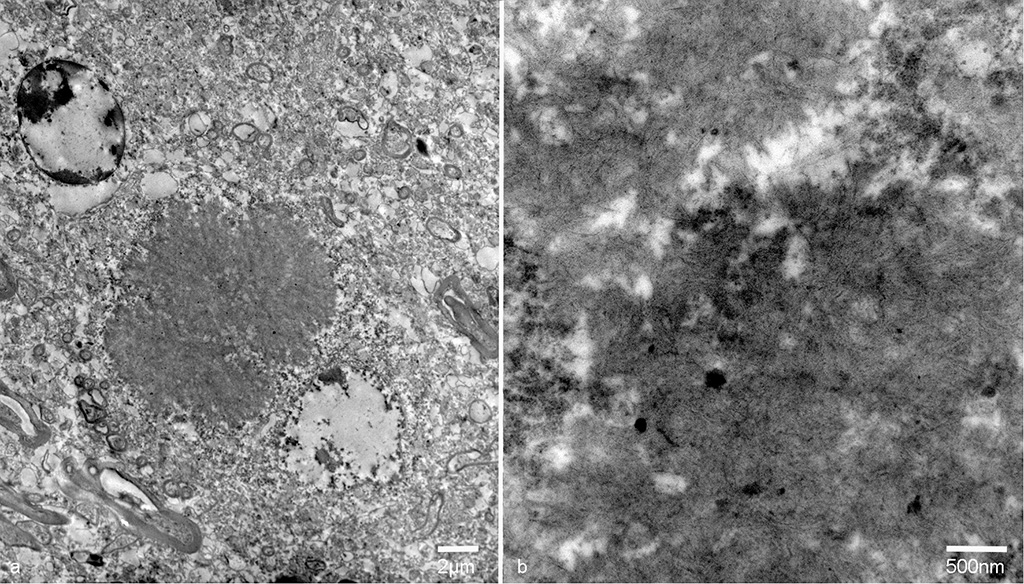
On transmission electron microscopy, the inclusion bodies in the neuronal perikarya and neuropils consisted of filamentous components. The width of the filaments was approximately 10nm. The inclusion bodies were not surrounded by membranes. Occasionally, filamentous components aggregated in the center and radially distributed to the periphery. The inclusion bodies in the neuropils were not surrounded by the myelin sheath.
Contributor’s Morphologic Diagnosis:
Diencephalon: Lafora-like bodies (polyglucosan bodies), intra-perikarya and intra-dendrites, numerous, Holstein, bovine
Contributor’s Comment: Several types of the inclusion bodies of the CNS have been recognized in humans and animals. The polyglucosan body is a one of these inclusions. In this case, the inclusion bodies seen in the CNS were considered to be polyglucosan bodies based on morphological and histochemical features. Polyglucosan bodies are classified into Lafora bodies and corpora amylacea based on their location in the tissue.6 Lafora bodies are localized in the perikarya and process of nerve cells; corpora amylacea are localized to within axons and /or astrocytes. In the present case, the inclusion bodies were primarily observed in perikarya, which identified them as Lafora bodies.
Lafora disease is a progressive myoclonic epilepsy in humans associated with an autosomal recessive hereditary defect.2,6 Histopathologically, the bodies were, amorphous to cauliflower-like, and some had core, slightly basophilic, cytoplasmic inclusion bodies, which are identified as Lafora bodies if seen in the CNS, heart, and liver. In animals, Lafora bodies in the CNS have been reported in dogs,8 cat,3 and cattle.5,9 Although hereditary spread has been reported in canines, the etiology of Lafora disease in cattle is obscure.
In addition, polyglucosan bodies in the CNS associated with aging have been reported in several animals.4,10 However, the present case was considered to be too young to have such age-related lesions. Although the genetic background and the association with age cannot be proven, the similarity of this case to human Lafora disease was considered interesting.
JPC Diagnosis: Pons, perikarya: Polyglucosan (Lafora) bodies, numerous, Holstein (Bos taurus), bovine.
Conference Comment: In humans, non-viral inclusion bodies have been further classified as Hirano, Pick, Lewy, Lafora, and Bunina bodies based on ultrastructural differences. Hirano-like bodies are more elongated microscopically and ultrastructurally are composed of beaded filaments. Bunina bodies are much smaller (2-5 µm) and are often arranged in clusters or chains. Bodies resembling Hirano and Bunina have been identified in horses and dogs, and although their pathogenic potential has not been investigated, there are a not uncommon feature of equine motor neuron disease. Finally, Lafora bodies are the most commonly identified non-viral inclusion in domestic animals and appear to be age related. They are located within neuronal parikarya, neuropil, and axons and are typically an incidental finding. These basophilic to amphophilic inclusions are strongly PAS-positive and metachromatic. Ultrastructurally, they are a mixture of branching filaments, non-membrane bound electron-dense bodies, and glycogen. Lafora bodies are generated due to abnormalities of carbohydrate metabolism which lead to the production of glucose polymers termed polyglucosans. When present in large numbers, they may be due to Lafora disease, which is analogous to the autosomal recessive form of human Lafora disease and has been reported in Basset Hounds, Poodles, Beagle dogs, and Miniature Wire-haired Dachshunds. In the Dachshund, it has the highest incidence and is a triplet repeat disorder (the first discovered in domestic animals).1 In a 2014 study, 50% of the study’s cohort carried at least one copy of the EPM2B mutation, which is one of the gene mutations in the human form of Lafora disease.7 In Lafora disease, the polyglucosan bodies are most numerous in Purkinje cells, and neurons of the caudate, thalamic, and periventricular nuclei. Clinically, these animals exhibit progressive myoclonus.1
Contributing Institution:National Institute of Animal Health,
National Agriculture and Food Research Organization (NARO)
3-1-5Kannondai, Tsukuba, Ibaraki 3050856, Japan
http://www.naro.affrc.go.jp/english/niah/index.html
References:
- Cantile C, Youssef S. Nervous system. In: Maxie, MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol. 1. 6th Philadelphia, PA: Elsevier; 2015:255, 292.
- Cavanagh JB. Corpora-amylacea and the family of polyglucosan disease. Brain Res Rev. 1999;29:265-295.
- Hall DG, Steffens WL, Lassiter L. Lafora bodies associated with neurologic signs in a cat. Vet Pathol. 1998;35:218-220.
- Kamiya S, Suzuki Y. Polyglucosan bodies in the brain of cat. J Comp Path. 1989;101:263-267.
- Kreeger JM, Frappier DC, Kendall JD. Systemic glycoproteinosis resembling Lafora’s disease in a cow. Cornell Vet. 1991;81:215-221.
- Minassian BA. Lafora’s disease: towards a clinical, pathologic, and molecular synthesis. Pediatr Neurol. 2001;25:21-29.
- Sainsbury R. DNA screening for Lafora's disease in miniature wire-haired dachshunds. Vet Rec. 2014; 175(22):568.
- Schoeman T, Williams J, Wilpe EV. Polyglucosan storage disease in a dog resembling Lafora’s disease. J Vet Intern Med. 2002;16:201-207.
- Simmons MM. Lafora disease in the cow. J Comp Path. 1994;110:389-401.?
- Yanai T, Masegi T, Iwanaka M, et al. Polyglucosan bodies in the brain of a cow. Acta Neuropathol. 1994;88:75-77.