CASE 4: W18-0154-4 (4132734-00)
Signalment:
Juvenile male red fox (Vulpes vulpes)
History:
This wild, juvenile, male red fox (Vulpes vulpes) was found moribund, lying on a storm drain cover in Washington, DC. The animal was thin, minimally responsive and had generalized alopecia with severe thickening, crusting and scaling of the skin, especially along the face, ears and dorsum. The fox was humanely euthanized.
Gross Pathology:
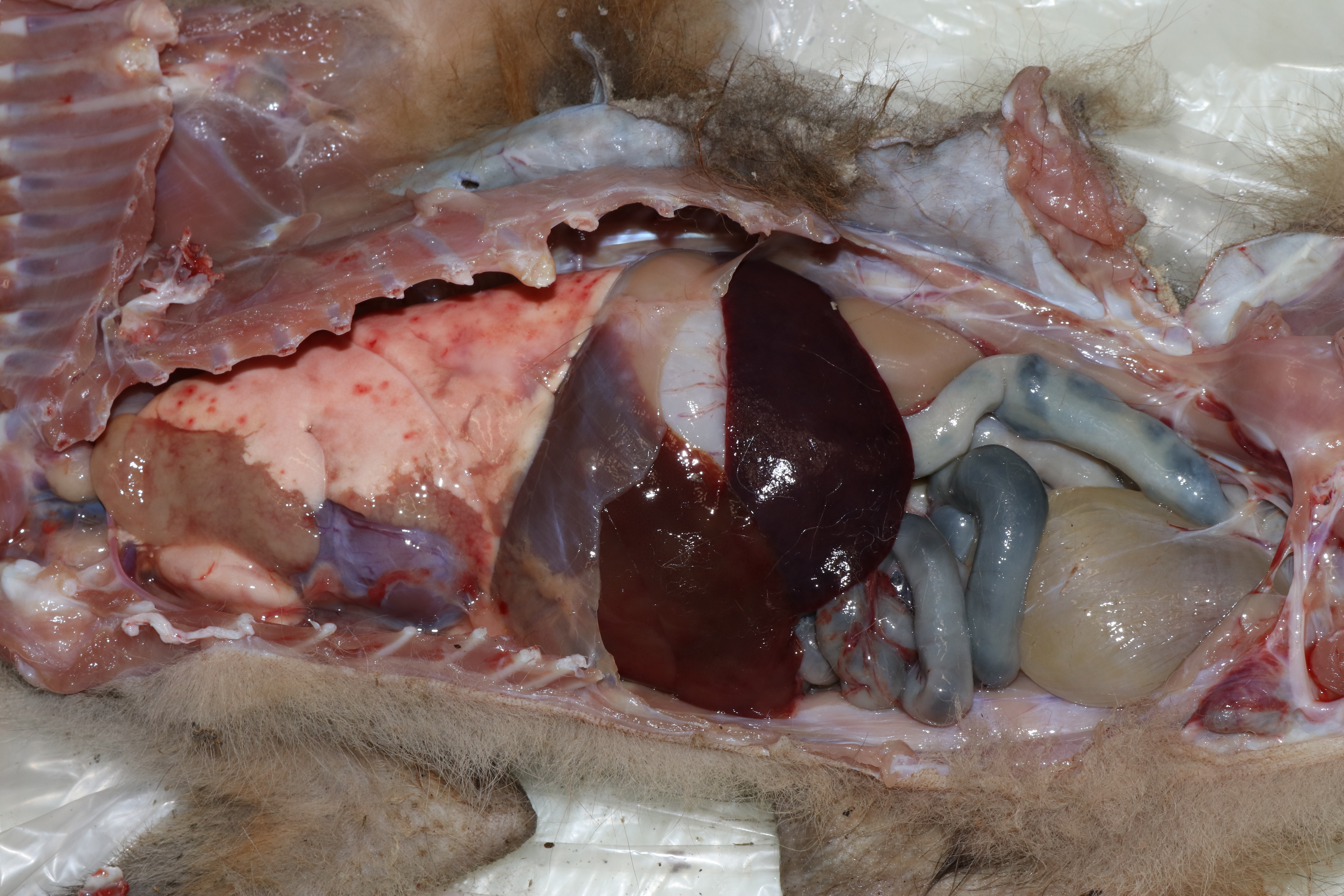
This fox was is in good postmortem condition and thin body condition (2.0 kg) with scant adipose stores and exaggerated bone prominences. The fur was multifocally thinned and the skin over most of the body was malodorous and markedly thickened by pale tan fissured crusts. The cranioventral portions of the left cranial and left caudal lungs lobes were consolidated, firm and pale brown. The right lung was mottled pink to dark red. In the free wall of the right ventricle of the heart, there was a 1 x 0.5 cm, well-demarcated focus of pale brown discoloration (euthanasia artifact). Small bronchi contained mucus and abundant, 0.5-1.5 cm long by < 1 mm diameter white worms. The stomach and small intestine contained mucoid dark brown to black material. The colon contained formed dark green-brown feces. No other gross lesions were appreciated.
Laboratory results:
Worms collected from the bronchi were examined by Dr. JM Kinsella, HelmWest Laboratory and were identified as Crenosoma vulpis based on the cuticular rings around the anterior end of males and the spicules, gubernaculum and bursal rays of the female.
The brain was negative for Rabies virus via direct immunofluorescence.
Microscopic description:
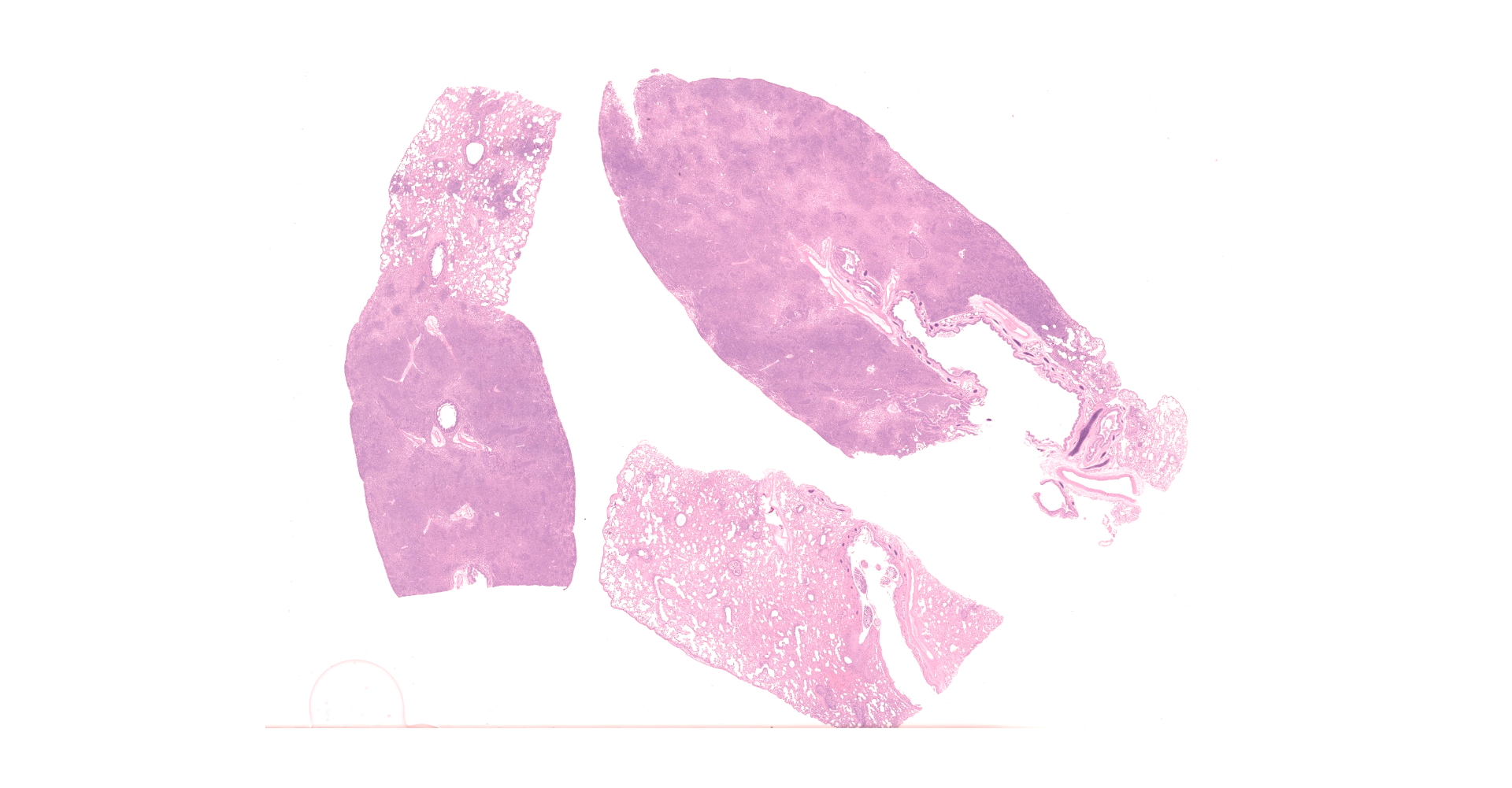
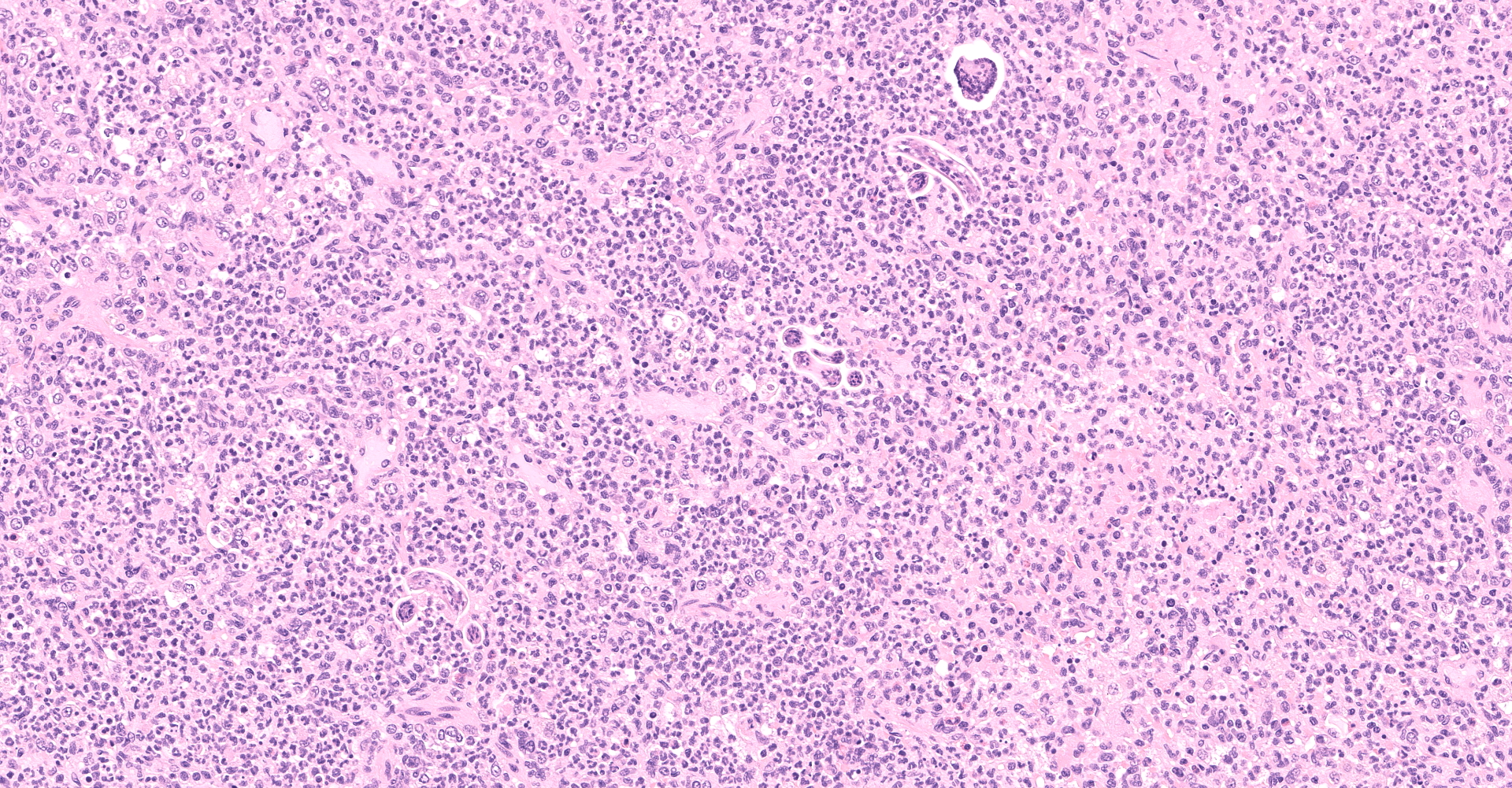
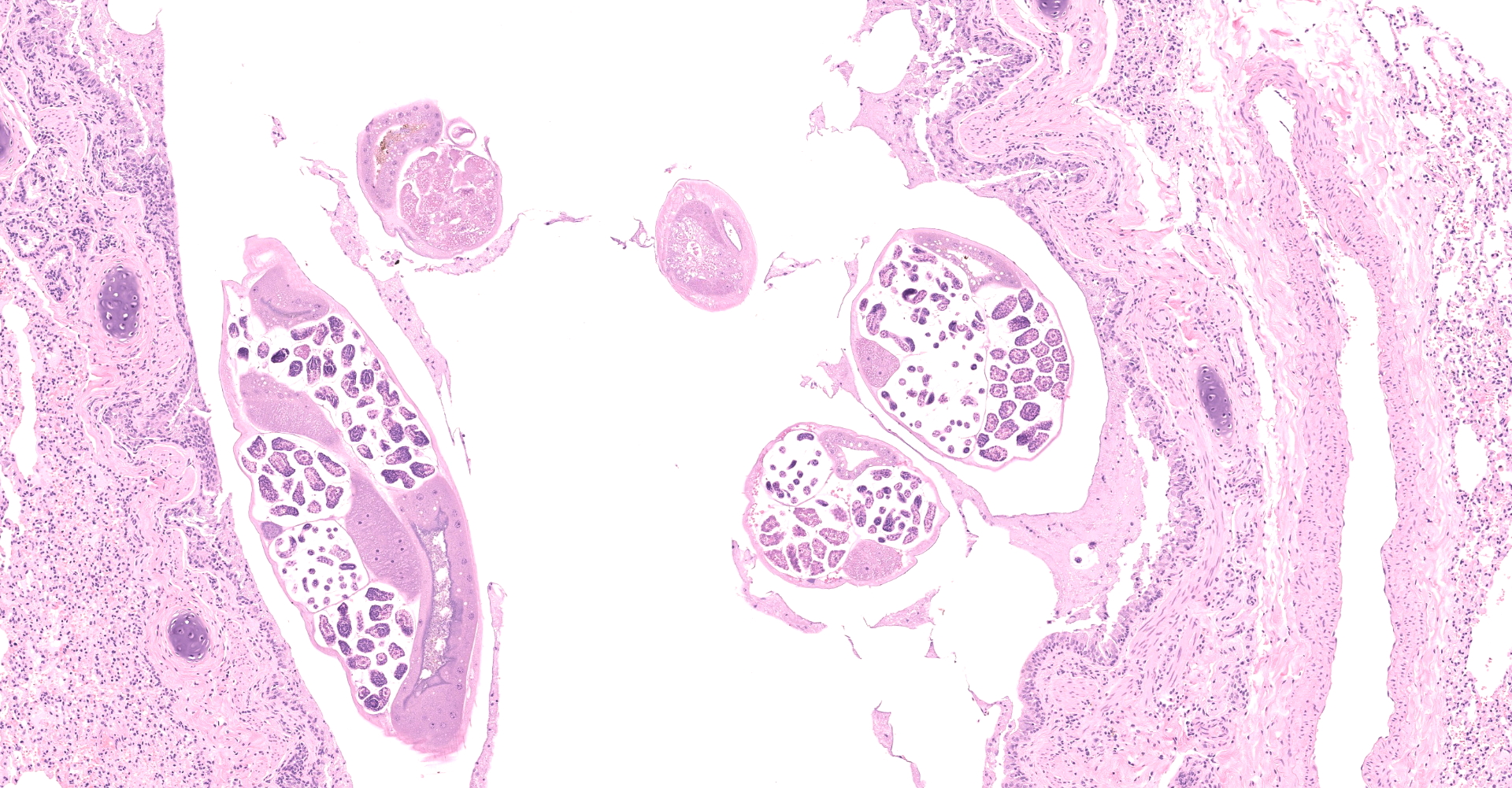
Multifocally to regionally, the normal architecture of the lung is obscured by a florid, basophilic, densely cellular infiltrate that often completely fills the air spaces of bronchioles and surrounding alveoli. Within alveoli, inflammatory cells consist predominantly of neutrophils and foamy macrophages with fewer multinucleated giant cells and eosinophils, that are admixed with proteinaceous fluid (edema) and two types of nematode eggs and free larval nematodes. The more numerous eggs are approximately 70 by 35 um and embryonated, with a thin to inapparent wall and a 10 to 12 um diameter, coiled larva with small lateral alae. These larvae are also free within alveolar lumina. The second, less numerous, egg type is embryonated (but not larvated), approximately 70 by 35 um, with bipolar plugs, a prominent, eosinophilic, 5 um thick wall containing ridges, and granular eosinophilic egg contents. Within the lumina of smaller bronchi, there are adult male (~200 um diameter) and female (~400 um diameter) nematodes that have a cuticle with thin longitudinal ridges, coelomyarian musculature, accessory hypodermal chords, a large intestine lined by few multinucleate cells and a genital tract containing either sperm or developing eggs with both embryos and first stage larvae present. In most (but not all) slides, within the epithelium of a larger bronchi, there is a 100 to 150 um diameter adult nematode with a thin cuticle, a pseudocoelom and hologonic gonads. Small airway (bronchioles and small bronchi) lumina are often filled and sometimes occluded by a mix of neutrophils, fewer eosinophils, free larvae and mucus. Few neutrophils and eosinophils transmigrate the respiratory epithelium, which often has increased goblet cells (mucus metaplasia) and plump and piled cells with large nuclei and vesiculate chromatin (bronchiolar/bronchial epithelial hyperplasia). Serous submucosal glands in a large airway are tortuous, dilated and filled with clear to slightly proteinaceous fluid. Moderate numbers of plasma cells and fewer lymphocytes infiltrate the adventitia of airways and pulmonary vessels.
Contributor's morphologic diagnosis:
Lung: Severe, regionally extensive, chronic, pyogranulomatous and eosinophilic bronchopneumonia with intrabronchial adult metastrongylid and aphasmid nematodes, intra-alveolar and intra-bronchiolar metastrongylid and aphasmid larvae and eggs, bronchiolar epithelial hyperplasia and mucus metaplasia.
Contributor's comment:
A dual lungworm infestation causing bronchopneumonia was diagnosed. Based on the morphology and location of eggs and adults described, the larger intraluminal adult worms, larva and thin-walled larvated eggs are consistent Crenosoma vulpis, while the smaller intra-epithelial adult worm and thick-walled eggs with bipolar plugs and ridges are consistent with Eucoleus aerophilus.9 Crenosoma vulpis identification was further confirmed based on examination of whole adult worms, which revealed cuticular ridges on the anterior end of males that is characteristic of this worm.
Crenosoma vulpis is a metastrongyle nematode known to infect domestic dogs and many wild carnivores in eastern North America and Europe, including red foxes, badgers (Meles meles), wolves (Canis lupus), raccoons (Pryocyon lotor)3 and Virginia opossum (Didelphis virginiana).7 The indirect life cycle involves the definitive mammalian host becoming infected when it eats a snail or slug (intermediate host) that harbors the third-stage larvae (L3).1 L3 larvae then penetrate the gastrointestinal tract wall and migrate to the lungs, where they mature into adults that live in the lumen of the trachea, bronchi or bronchioles.1 Thin-shelled eggs containing larva or free larvae deposited by adults become lodged in alveoli, and migrate up the airways until they are coughed up, swallowed and passed in the feces where they can infect the intermediate host.1,3
Eucoleus aerophilus is an aphasmid nematode that was first described in 1839 and named Trichiosoma aerophilus. Subsequent name and taxonomic designation changes (formerly Thominx aerophilus and Capillaria aerophila) have landed the organism in the Eucoleus genus.2 Documented infestations have occurred in wildlife, domestic cats and humans in a wide geographic range.8 Much of the biology and life history of this parasite is still poorly understood. E. aerophilus is thought to have a direct life cycle, where adults living in the epithelium of the respiratory tree deposit eggs that are coughed up, swallowed and passed in the feces.3,13 Eggs may develop directly in the environment or be ingested by earthworms, which act as a facultative intermediate host.3,13 Characteristic histologic features of aphasmids are bacillary bands and a stichosome, which were not visible in the few sections of adult worms in this case. But based on the size and location of adult worms (intra-epithelial in a bronchus), and presence of thick-walled eggs with bipolar plugs, Eucoleus aerophilus is considered most likely.
C. vulpis and E. aerophilus infestations are important causes of chronic pulmonary disease in both wild and domestic canids, especially in the northeastern United States and Canada where dual infestations by both worms are common in wild red foxes.10 Clinically, infected animals often have a chronic cough and resemble animals afflicted with allergic bronchitis and chronic obstructive pulmonary disease (COPD).10 Pathologic changes within the lungs of wild foxes are typically limited to airway-centered exudate (eosinophilic and catarrhal bronchitis/ bronchiolitis), BALT hyperplasia, submucosal bronchial gland hyperplasia, smooth muscle hypertrophy of airways and mucus metaplasia.5,10 C. vulpis tends to inhabit smaller airways (small bronchi and bronchioles), whereas E. aerophilus prefers larger bronchi, as was the case in this fox.10 Granulomatous pneumonia can occur in canids when C. vulpis larvae are aspirated into alveoli and bacterial pneumonia can complicate both types of infestations.5 In dogs experimentally infected with C. vulpis, a marked granulocytic exudate filled alveoli (in addition to terminal bronchioles) in the early stages of infection as L3 larvae migrated into the lungs.12 The heavy involvement of alveoli and lack of significant smooth muscle hypertrophy and submucosal gland hyperplasia in this case may reflect a more acute and fulminant course of disease in a juvenile animal with an immature immune system and severe concurrent ectoparasitism (this animal was also heavily infested with sarcoptic mange and in poor body condition at the time of euthanasia).
Metazoan parasites of importance in the respiratory tract of carnivores that must be distinguished from C. vulpis and E. aerophilus include: Aelurostrongylus abstrusus, which is similar to C. vulpis but parasitizes felids5; Angiostrongylus vasorum that reside the pulmonary arteries5; Eucoleus (=Capillaria) boehimi that reside in the nasal cavity and have smaller (50-60 x 30-35 um) eggs with a pitted rather than ridged wall6; Paragonimus kellicotti, which is a trematode5; and Oslerus (=Filaroides) osleri, which typically cause grossly evident submucosal nodules in the trachea and bronchi5; Andersonstrongylus (=Filaroides) milksi and Filaroides hirthi. Andersonstrongylus milksi and Filaroides hirth are metastrongyles that are similar in size to C. vulpis and also inhabit the bronchioles, thus differentiation of these three worms based on histology is problematic and examination of intact adults is preferred (as was done in this case).
This case highlights the importance of a thorough histologic evaluation with special attention paid to secondary or tertiary diseases that may be present in examined organs. Although parasite morphology and identification can at times be challenging for a pathologist, when multiple parasite lifestages are present care should be taken to ensure they do not represent separate species.
Contributing Institution:
Smithsonian Institution's National Zoological Park
www.nationalzoo.si.edu
JPC diagnosis:
Lung: Bronchopneumonia, pyogranulomatous, chronic, multifocal to coalescing, severe, with intraluminal metastrongyle and intraepithelial aphasmid adults, and intra-alveolar larvae, and metastrongyle and aphasmid eggs.
JPC comment:
The contributor provides a very thorough analysis of this case, and well illustrates the capacity of animals to have coinfections pathogenic organisms, nematodes in this case. As the contributor stated, C. vulpis and E. aerophilus have a wide geographic distribution. In particular, C. vulpis, E. aerophilus, and Angiostrongylus vasorum are particularly problematic for domestic dogs and wild canids, particularly red foxes, in central Germany. Across three Federal states (Thuringia, Rhineland-Palatinate, Hesse), 569 red foxes examined had prevalence of C. vulpis infections of up to 32.3%, A. vasorum of up to 14.1%, and E. aerophilus of up to 69.4%. In the examined red foxes, approximately 30.7% had dual infections with E. aerophilus and C. vulpis. A small subset of foxes (5%) had triple infections, with A. vasorum in addition to C. vulpis and E. aerophilus.11
Recent investigations of lungworm infections in red foxes have used geographic information systems (GIS) to visualize the cases, allow for geospatial analysis such as cluster analysis, and to attempt correlation of cases with other environmental factors.4,11 Investigation in Slovakian red foxes found moderately good predictive value in a multivariate model using backward stepwise regression. The model indicated that infection of A. vasorum is positively correlated with areas of lower warm period precipitation and a higher share of arable land and permanent cultures.4
References:
1. Anderson R. Order Strongylida. In: Nematode Parasites of Vertebrates: Their Development and Transmission. Oxon, UK: CAB International; 1992:35?208.
2. Bowman A. Eucoleus aerophilus. American Association of Veterinary Parasitologists. 2014: retrieved from http://www.aavp.org/wiki/nematodes/aphasmidida/eucoleus-aerophilus/
3. Bowman DD. Georgis' Parasitology for Veterinarians, 10th Edition. St. Louis, MO: Elsevier; 2014.
4. Cabanova V, Miterpakova M, Druga M, Hunikova Z, Valentova D. GIS-based environmental analysis of fox and canine lungworm distributions: an epidemiological study of Angiostrongylus vasorum and Crenosoma vulpis in red foxes from Slovakia. Parasitology Research. 117:521-530.
5. Caswell J, Williams K. Respiratory System. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. St. Louis, MO: Elsevier; 2016:465?591.
6. Conboy G. Helminth Parasites of the Canine and Feline Respiratory Tract. Vet Clin North Am Small Anim Pract. 2009 Nov;39:1109?1126.
7. K Rice J. Crenosoma vulpis larva (fox lungworm) in a juvenile opossum from Northern Virginia, USA. Int Int J Avian Wildl Biol. 2018 Apr 2;3:4?2.
8. Lalo?evic D, Lalo?evic V, Klem I, Stanojev-Jovanovic D, Pozio E. Pulmonary Capillariasis Miming Bronchial Carcinoma. Am J Trop Med Hygeine. 2008;78:14?16.
9. Lalo?evi? V, Lalo?evi? D, ?apo I, Simin V, Galfi A, Traversa D. High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia. Parasite. 2013;20.
10. Nevárez A, López A, Conboy G, Ireland W, Sims D. Distribution of Crenosoma Vulpis and Eucoleus Aerophilus in the Lung of Free-Ranging Red Foxes (Vulpes Vulpes). J Vet Diagn Invest. 2005 Sep;17:486?489.
11. Schug K, Kramer F, Schaper R, et al. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vuples vulpes) in central Germany. Parasites and Vectors. 2018;11:85.
12. Stockdale PHG, Hulland TJ. The Pathogenesis, Route of Migration, and Development of Crenosoma vulpis in the Dog. Pathol Vet. 1970 Jan 1;7:28?42.
13. Traversa D, Di Cesare A, Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit Vectors. 2010 Jul 23;3:62.