Wednesday Slide Conference, Conference 2, Case 3
Signalment:
Fourteen-week-old, female, British shorthair cat (Felis catus).
History:
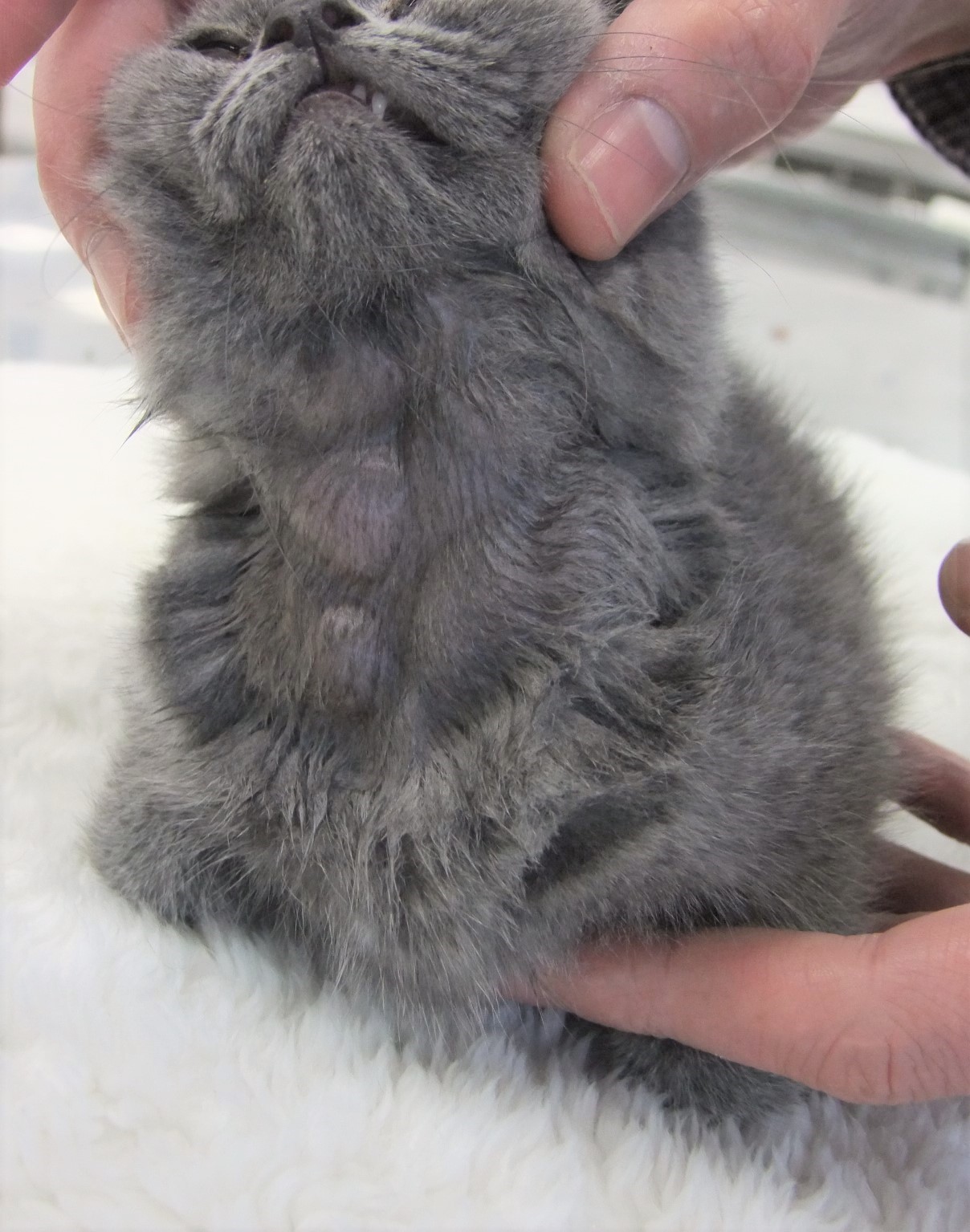
This kitten was one of two in a litter of four British shorthair (BSH) kittens that developed multicentric lymphadenopathy involving all peripheral lymph nodes at 6 weeks of age. Over the following weeks, the lymphadenopathy rapidly progressed to marked but non-painful enlargement of multiple nodes with progressive abdominal distension and lethargy (Figures 1 and 2). Both kittens showed mild regenerative anemia, and blood smears from this kitten showed gross auto-agglutination. Both kittens received immunosuppressive doses of corticosteroids (2.2mg/kg prednisone per os sid) for the 2 weeks prior to euthanasia, but no significant clinical improvement was observed and they were euthanized at 12 and 14 weeks respectively.
Gross Pathology:
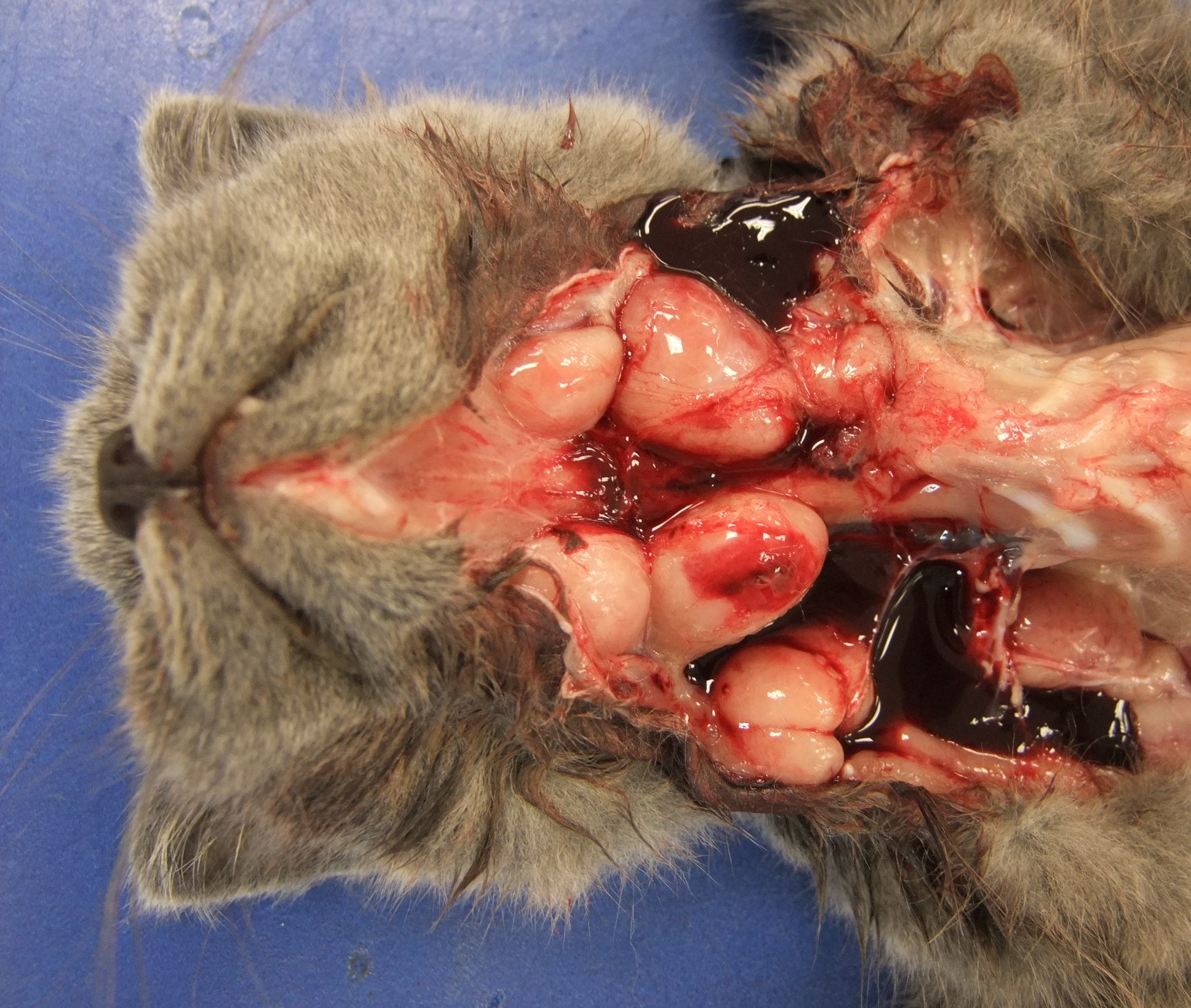
On necropsy examination, all identifiable peripheral and visceral lymph nodes showed very marked enlargement (Figures 3 and 4) with effacement of corticomedullary architecture when incised (Figure 5). Mild diffuse hepatic enlargement and moderate diffuse splenic enlargement were also present.
Laboratory Results:
Blood was negative for the presence of both FeLV antigen and FIV antibody (Snap® Combo FeLV Ag/FIV Ab Test Kit) and was negative on direct Coombs’ testing. Immunocytochemistry to assess CD3, CD4 and CD8 expression performed on fine needle aspirates from multiple lymph nodes indicated a CD3+/CD4/CD8- immunophenotype for the majority of cells. Results of molecular clonality PCR amplification of antigen receptor rearrangements (PARR) of both the T-cell receptor gamma (TCRG) and immunoglobulin heavy chain (IGH) loci on genomic DNA extracted from FFPE lymph node tissue was consistent with a polyclonal and non-neoplastic T-cell proliferation. DNA extracted from fresh-frozen kidney and tested by PCR revealed homozygous Fas-ligand gene (FASLG) variants associated with feline autoimmune lymphoproliferative syndrome (FALPS).
Microscopic Description:
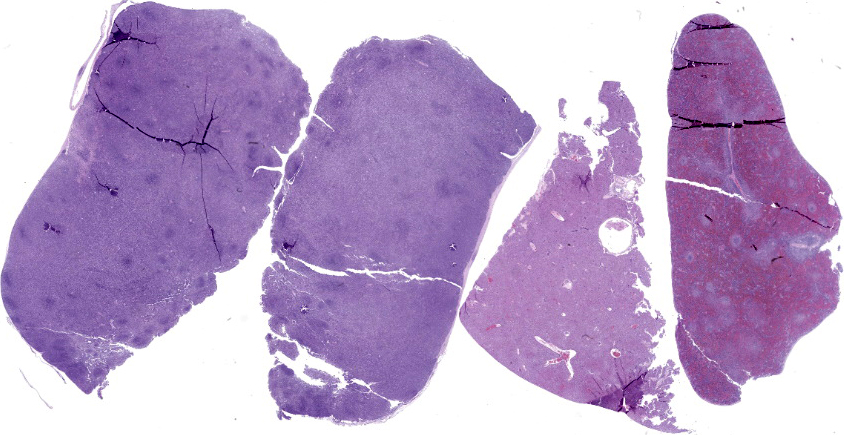
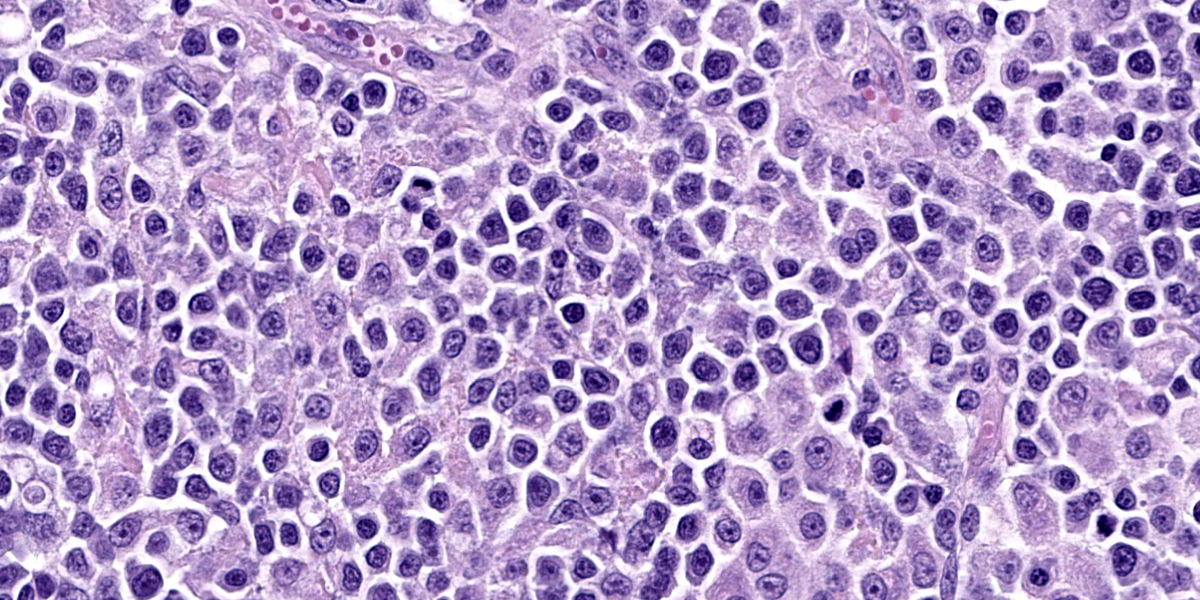
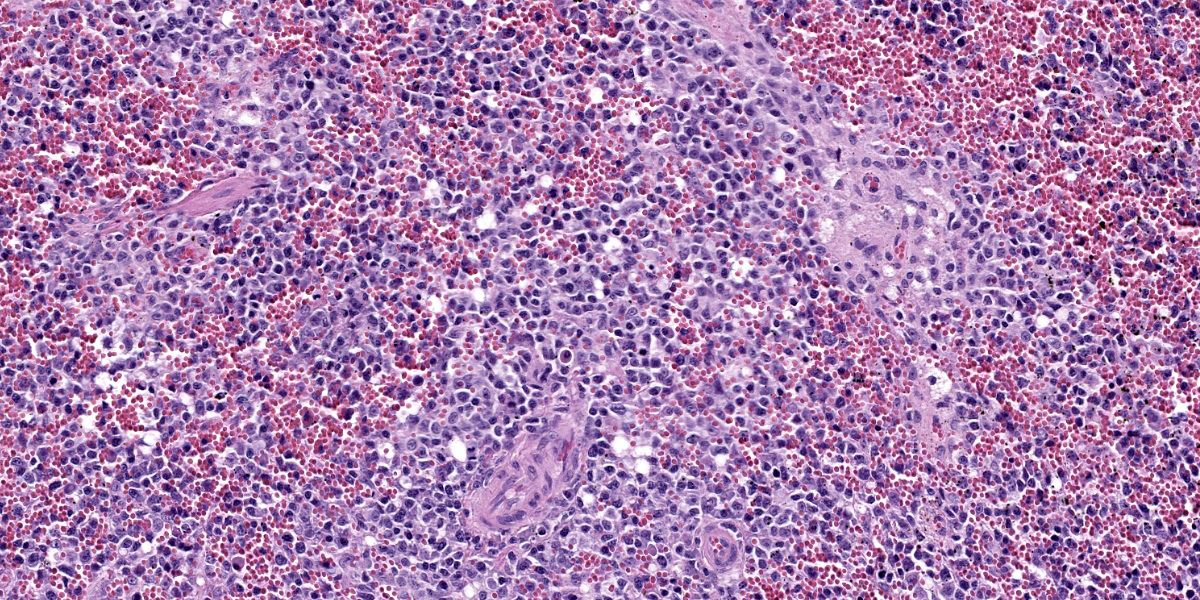
Lymph nodes: There is marked expansion of the cortex and medulla of both nodes by a population of round cells consistent with lymphocytes, which effaces or markedly distorts nodal architecture and follicular remnants and expands subcapsular sinuses (Figure 6). Lymphocytes are intermediate to large in size, have generally distinct borders with a scant to moderate amount of eosinophilic cytoplasm, large round nuclei with clumped chromatin and one to two prominent nucleoli. The nuclear diameter of lymphocytes is typically equal to the diameter of approximately 1.5-2 regional erythrocytes. Mitotic figures average 8-10 per 400x high power fields. In some areas, low to moderate numbers of small lymphocytes, plasma cells, macrophages and rare neutrophils are also admixed with the lymphocyte population (Figure 7).
Spleen: There is moderate multifocal expansion of the splenic white pulp by a population of lymphocytes similar to those present and described within the lymph nodes.
Liver: There is mild multifocal expansion of periportal areas and more variably, hepatic sinusoids, by a population of cells including both small and large lymphocytes, plasma cells (including Mott cells), and macrophages. Multifocal areas of scattered extramedullary hematopoiesis are present throughout the section and low numbers of Kupffer cells contain intracytoplasmic erythrocytes.
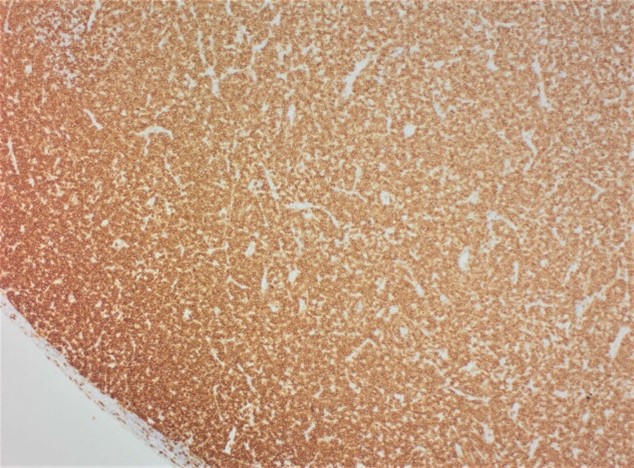
Immunohistochemistry for CD3 and CD20 performed on sections of lymph nodes and spleen confirmed a predominantly CD3+/CD20- cell population consistent with T cells within both tissues (Figure 8), while variable numbers of CD3-/CD20+ B-cells were also present within follicular remnants and subscapular sinuses of some nodes. This staining pattern is consistent with a T-cell proliferation.
Contributor’s Morphologic Diagnosis:
Lymph nodes, spleen and liver: T-cell lymphoid hyperplasia, severe, diffuse
Liver: Pericholangitis and perivasculitis, mild, multifocal, lymphoplasmacytic with erythrophagocytosis and extramedullary hematopoiesis
Contributor’s Comment:
This case illustrates typical features of feline autoimmune lymphoproliferative syndrome (FALPS), an unusual autosomal recessive lymphoproliferative disease first seen in multiple related British shorthair kittens in Australia in the 1990s and in New Zealand from 2008.2 Kittens affected by FALPS appear normal at birth but show failure to thrive, lethargy, regenerative anemia, abdominal distension and generalized lymphadenopathy from 6-10 weeks of age. The disease progresses quickly, has no known effective treatment, and affected kittens usually die or require euthanasia shortly after diagnosis. The disease is easily misdiagnosed as lymphoma, as gross pathology, routine histology and immunohistochemistry results all suggest a diagnosis of a neoplastic (T-cell) proliferation in multiple lymph nodes, spleen and other organs. However, PCR molecular clonality assays (PARR) confirm a polyclonal and non-neoplastic T-cell proliferation within affected nodes indicative of a hyperplastic process and inconsistent with lymphoma.
The genetic basis for FALPS has recently been identified as a monogenic autosomal recessive mutation in the Fas-ligand gene (FASLG).3 Both the FAS and FASLG genes code for proteins critical in normal cell apoptosis. The mutation in kittens with FALPS involves the insertion of an adenine base in exon 3 of FASLG, causing a frameshift mutation and insertion of a premature stop codon, predicted to produce a truncated Fas ligand protein that is unlikely to initiate effective lymphocyte apoptosis. Kittens homozygous for the FASLG variant allele develop FALPS while heterozygotes are carriers of the defect but phenotypically normal. Genetic testing (buccal swabs or blood) is currently available through Massey University (New Zealand) and Langford Vets (United Kingdom). Recent studies show a relatively high frequency of the variant FASLG allele in BSH cats in New Zealand, with 22% of healthy BSH cats from three breeding catteries identified as carriers of the FASLG variant.1 The disease is not currently reported outside Australasia, but as breeding BSH cats from New Zealand and Australia are often exported, it is possible that FALPS may also be seen in BSH and BSH-cross cats in other countries.
The disease in BSH cats is analogous to the inherited disease autoimmune lymphoproliferative syndrome (ALPS) in people.8 The majority of people with ALPS have inherited FAS gene mutations causing defective lymphocyte apoptosis, non-neoplastic lymphoproliferation and variable autoimmunity, although mutations in both FASLG and caspase 10 genes can also cause the disease. In people, most ALPS cases have autosomal dominant inheritance, but the genotype often shows incomplete penetrance with a variable phenotype. Feline autoimmune lymphoproliferative syndrome appears similar to a rare autosomal recessive ALPS variant in people with homozygous FASLG mutations which causes a severe and often fatal form of the disease (“ALPS-FASLG”) in children.6 The feline disease also shows similarities to the autosomal recessive “gld” mouse model for ALPS, where mice with homozygous FASLG mutations develop severe lymphoproliferative disease early in life.7
As this case illustrates, the main gross and histological differential diagnosis for FALPS is multicentric lymphoma, and early cases of FALPS were misdiagnosed as this. Cytology (not shown here) and histology of multiple enlarged lymph nodes in this kitten reveal a monomorphic population of large lymphocytes with a high mitotic rate. Lymphocytes efface or markedly distort normal nodal architecture and fill subcapsular sinuses. These features are strongly suggestive of a neoplastic lymphoid proliferation. In most FALPS cases, as here, low numbers of randomly distributed plasma cells, macrophages and neutrophils also present in some node sections, while other cases show less complete effacement and more obvious retention of cortical architecture. Similar but less dramatic lymphoproliferation is also consistently seen within the spleen (as in this case), and more variably within the liver and gastrointestinal tract in kittens with FALPS. The majority of lymphocytes within lymph nodes and spleen typically show a CD3+ immunophenotype (consistent with T-cells), also suggesting (T-cell) lymphoma. Immunocytochemistry shows these lymphocytes to be an unusual population of “double negative T-cells” (CD3+/CD4-/CD8-), also similar to those seen in people with ALPS.8 Mild erythrophagocytosis and extramedullary hematopoiesis, possibly secondary to hemolytic anemia, are also seen in the liver in this case; in other FALPS cases these histological features are more marked. The milder erythrophagocytosis and extramedullary hematopoiesis in this case may be related to this kitten’s corticosteroid treatment prior to euthanasia; corticosteroids are the first-line of therapy in people to reduce the autoimmune manifestations of ALPS, including anemia.10
Despite microscopic features suggesting T-cell lymphoma in this kitten, PARR testing of lymph node samples showed a polyclonal non-neoplastic T-cell proliferation within lymph nodes and other lymphoid tissues, as is characteristic in FALPS-affected kittens. Subsequent genetic testing also confirmed the presence of the homozygous FASLG mutations now known to be associated with FALPS.3 Lymphoma should also be considered an unusual diagnosis in such a young kitten (FALPS-affected kittens are usually less than 5 months of age), particularly where multiple related or sibling kittens are affected by the disease. Although lymphoma has been reported in kittens as young as 9 weeks of age following experimental FeLV infection at birth,4 lymphoma in kittens under 6 months of age following natural infection appears very rare. FALPS should therefore be considered as a potential differential diagnosis in any BSH or BSH-cross kitten under 5 months of age with enlargement of multiple peripheral lymph nodes, especially where multiple related animals are affected by disease.
Contributing Institution:
Massey University
School of Veterinary Science
Private Bag 11 222
Palmerston North 4442
New Zealand
JPC Diagnosis:
Lymph nodes, spleen: Atypical lymphoid proliferation, diffuse, severe.
Liver: Extramedullary hematopoiesis, multifocal, mild to moderate.
JPC Comment:
In case you thought we lost our minds (we haven’t, but thanks for asking!) and put the same entity within the same conference as a separate case, you can be at ease. While this entity may look like lymphoma at first glance, the contributor provides an excellent slide description and summary of FALPS and highlights how a good history and ancillary diagnostics are needed to recognize this entity and spot the differences. That the animal in this case was only 14 weeks old is important – we actually put this detail on the Conference Worksheet to help push participants away from lymphoma and open the door to thinking about other possible diagnoses. Although some conference participants listed FALPS as their associated condition for this case, other rule outs considered were myeloma and FELV-associated lymphoma though the expected time course of these neoplasms do not fit the age of the animal in this case well. The atypical lymphocyte morphology in this case is similar to the lymphocyte morphology in Case 1 with regards to size and chromatin patterns – that these cells were noted in the red and white pulp of the spleen and lymph node led some participants to still favor lymphoma for this case. Although subtle, the low to moderate numbers of inflammatory cells in the background are not an expected feature of lymphoma the same way that they were in the intestine in Case 1. Notably, there are plasma cells and even numerous Mott cells that multiple conference participants pointed out. We ran IHCs for CD3, CD20, PAX5, MUM1, and IBA1. Lymphocytes did not label with B-cell markers (PAX-5 and CD20) consistent with the contributor’s assessment of CD3 reactivity for these cells of interest. IBA1 was not particularly helpful in this case as it labeled the normal existing population of histiocytes within the spleen, liver, and lymph nodes quite well. These findings altogether highlight the validity of PARR to distinguish FALPS from lymphoma as noted by the contributor.
The present entity circles back to general pathology and apoptosis rather nicely. In the extrinsic pathway of apoptosis, plasma membrane receptors (so called ‘death receptors’) respond to changes in the extracellular environment by trimerizing in response to ligand presentation.3,5,9 Examples of death receptors include members of the tumor necrosis factor superfamily (e.g.TNFR1) and FS-7-associated surface antigen (FasR) among others.5 After the ligand-receptor binding interaction, propagation of this signal continues within the cell via continues via associated death domain proteins (FADD/TRADD) which complex with pro caspase-8 to form a death induced signaling complex (DISC).5,9 The net effect of this interaction is that the death domain protein serves to activate caspase 8 at an appropriate time and initiate a death cascade via downstream ‘executioner’ caspases with caspase 3 being the most significant. These caspases cleave nuclear and cytoplasmic proteins, leading to disintegration of the nucleus and disruption of the cytoskeleton during apoptosis.5 Connecting these details back to the case at hand, Fas ligand is normally expressed on a variety of cells including T-lymphocytes – FasL therefore acts as brake of sorts on an excessive immune response. As the contributor points out, a frameshift mutation in the Fas-ligand gene would be expected to disrupt FasL and FasR interaction and lead to a non-neoplastic accumulation of lymphocytes due to lack of downstream caspase activity and lack of apoptosis. Conference participants remarked about the presence of tingible body macrophages within lymphoid follicles in this case despite this mutation and felt that alternate death receptors in the TNF superfamily might still be active and allow some lymphocytes to be sufficiently phosphorylated to activate downstream caspases.
Finally, testing for this rare condition has expanded in recent years. In Europe, commercially available testing for FALPS is included with genetic screening for polycystic kidney disease and progressive retinal atrophy in British Short/Longhair cats via Laboklin. As the popularity of British Shorthair cats increases in the United States, demand for such testing may also follow.
References:
- Aberdein D, Munday JS, Dittmer KE, Heathcott RW, Lyons LA: Frequency of a FAS ligand gene variant associated with inherited feline autoimmune lymphoproliferative syndrome in British shorthair cats in New Zealand. New Zealand Veterinary Journal. 2017:1-5.
- Aberdein D, Munday JS, Fairley RA, Vernau W, Thompson KG: A novel and likely inherited lymphoproliferative disease in British shorthair kittens. Veterinary Pathology. 2015:52(6):1176-1182.
- Aberdein D, Munday JS, Gandolfi B, Dittmer KE, Malik R, Garrick DJ, et al.: A FAS-ligand variant associated with autoimmune lymphoproliferative syndrome in cats. Mamm Genome. 2017:28(1-2):47-55.
- Hoover EA, Perryman LE, Kociba GJ: Early lesions in cats inoculated with feline leukemia virus. Cancer Res. 1973:33(1):145-152.
- Miller MA, Lyle LT, Zachary JF. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. St. Louis, MO: Elsevier; 2022:28-29.
- Nabhani S, Honscheid A, Oommen PT, Fleckenstein B, Schaper J, Kuhlen M, et al.: A novel homozygous Fas ligand mutation leads to early protein truncation, abrogation of death receptor and reverse signaling and a severe form of the autoimmune lymphoproliferative syndrome. Clinical Immunology. 2014:155(2):231-237.
- Nagata S, Suda T: Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995:16(1):39-43.
- Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010:116(14):e35-40.
- Santagostino SF, Assenmacher C-A, Tarrant JC, Adedeji AO, Radaelli E. Mechanisms of Regulated Cell Death: Current Perspectives. Veterinary Pathology. 2021;58(4):596-623.
- Worth A, Thrasher AJ, Gaspar HB: Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. British Journal of Haematology. 2006:133(2):124-140.