Signalment:
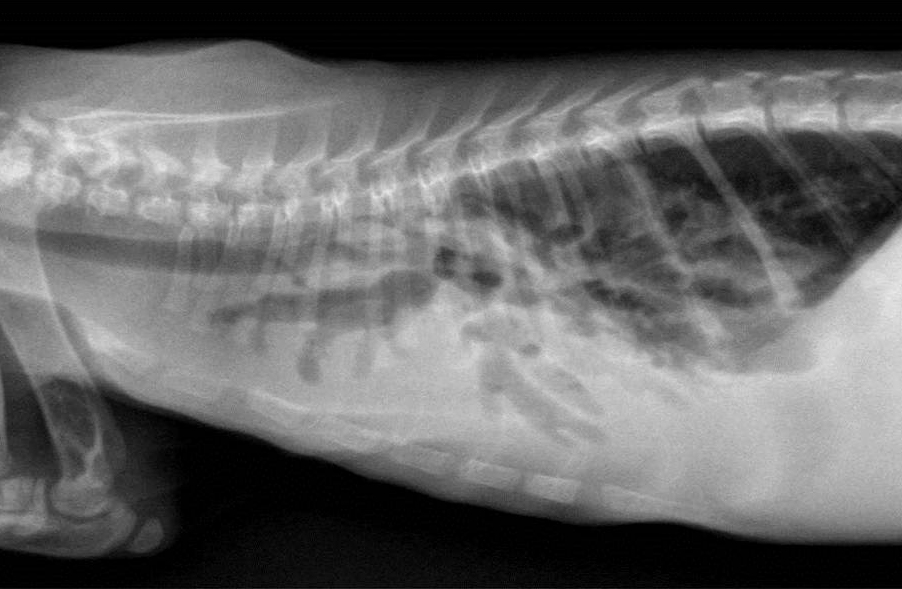
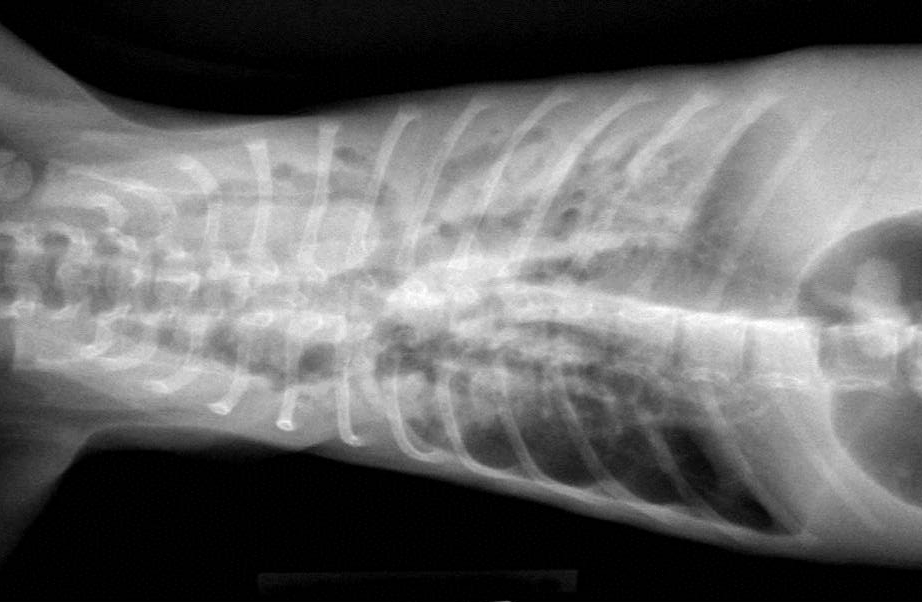
Gross Description:
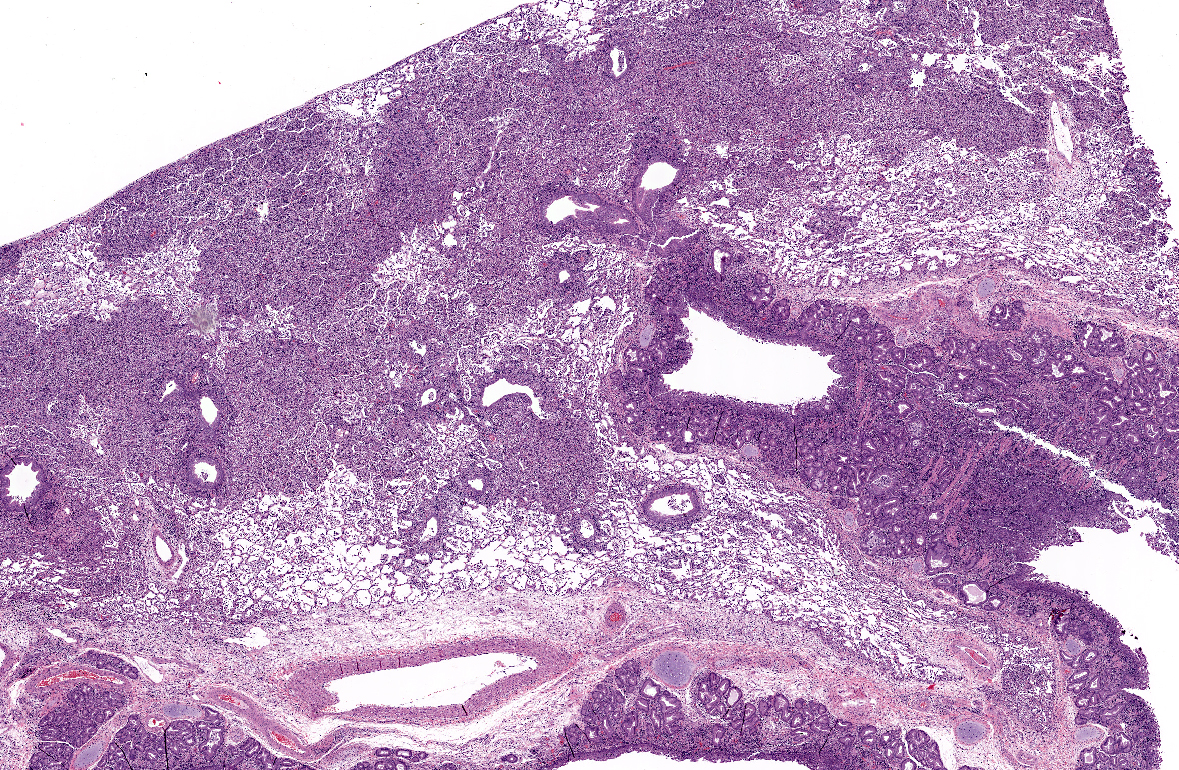
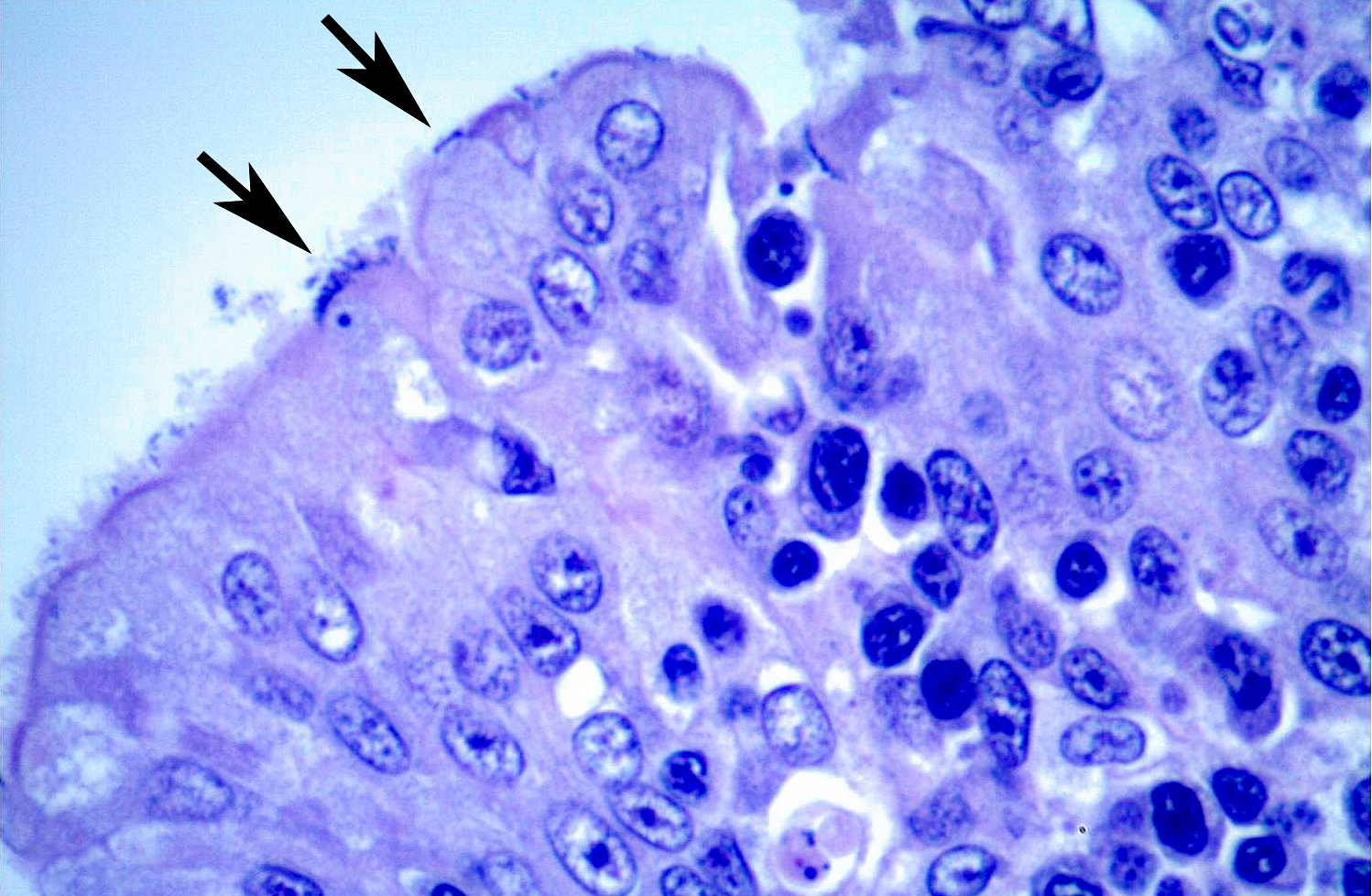
Histopathologic Description:
A moderate lymphoplasmacytic infiltrate is present in the submucosa of the bronchi and trachea, which is further expanded by edema. Multifocally there is erosion and ulceration of the epithelium covered by a thick layer of fibrin admixed with degenerate neutrophils.
Right caudal lobe (not submitted with case material): The section examined is nearly free of pathology with delicate alveolar walls and normal bronchioles. Multifocally there are small aggregates of neutrophils in alveolar and perivascular spaces. Scarce neutrophils and macrophages are present in alveolar spaces.
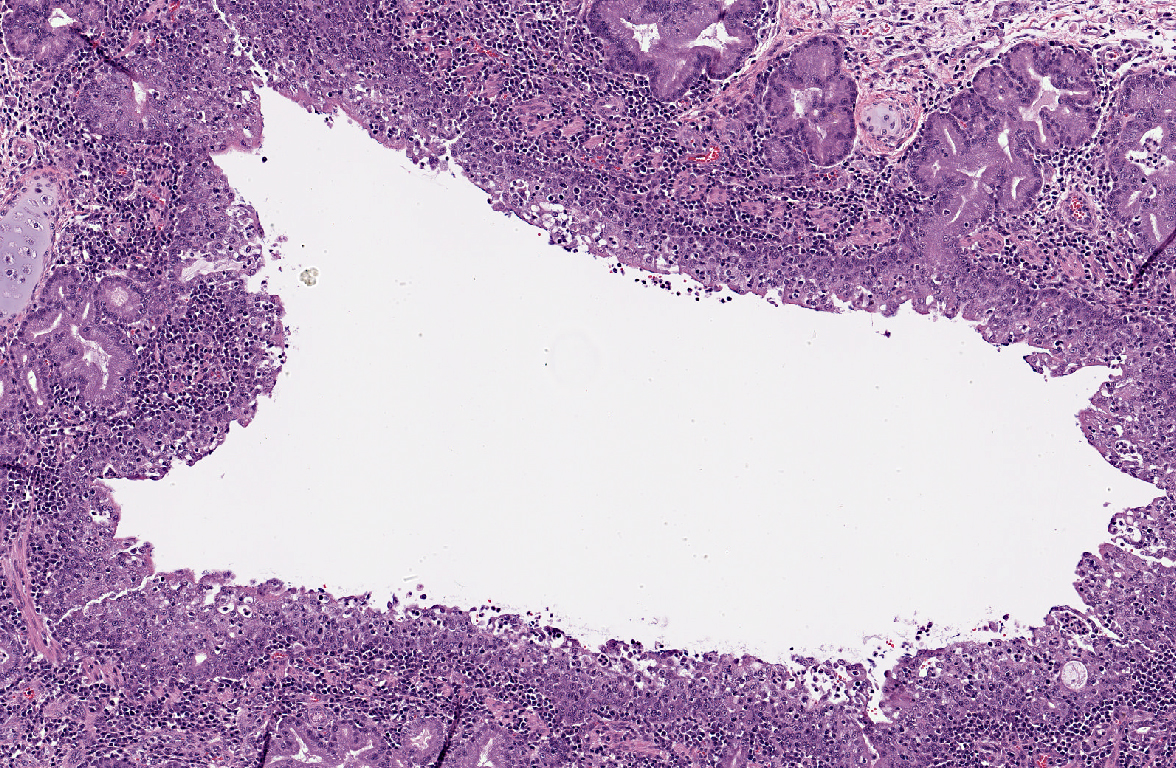
Tracheobronchial lymph node (slide 11, not present on all slides): Medullary sinuses and cords are expanded by numerous histiocytes. Multifocally there are discrete clear spaces containing tingible body macrophages. Numerous lymphocytes have pyknotic and fragmented nuclei (lymphocytolysis).
Morphologic Diagnosis:
Lab Results:
Clinical tracheal wash cytology:
-�-� Highly cellular, numerous nondegenerate neutrophils, few macrophages and rare well-differentiated anucleated squamous cells.
-�-� Heterogenous population of extracellular bacteria, including diplococci, rods, small rods in chains, and possibly Mycoplasma organisms, is noted.
-�-� Interpretation: Marked neutrophilic inflammation
Clinical tracheal wash culture: moderate mixed growth of oral flora
Blood chemistry:
-�-� PCV/TP - 35%/7.4 g/dL
-�-� Blood glucose - 89 mg/dL
-�-� BUN (azo) 5-15
Post-mortem aerobic culture of lung tissue: heavy growth Mycoplasma sp (not speciated)
Viral PCR on lung tissue:
-�-� Negative for Chlamydia, feline herpes virus, influenza A
-�-� Weakly positive for feline corona virus (CT value 29)
-�-� Weakly positive for feline calici virus (CT value 38)
Condition:
Contributor Comment:
Mycoplasma species are common contributors to lower respiratory disease as single or contributory agents in pigs, cattle, and humans. In cats, Mycoplasma spp are considered by many to be normal flora of the upper respiratory tract that can contribute to upper respiratory signs and ocular diseases. A single case report of a cat with primary, culminant pneumonia requiring mechanical ventilation is reported(9). Three cats are included in a retrospective review of 17 cases of Mycoplasma respiratory infections in small animals at Colorado State(5). In a retrospective study of lower respiratory infections in cats, 11 of 18 were attributed to primary Mycoplasma infection, another case was attributed to Mycoplasma and Pasteurella multocida coinfection, and the remaining 6 individual cases were attributed to various non- Mycoplasma bacterial, fungal and parasitic causes(5).
Feline calicivirus is a common cause of both upper and lower respiratory disease in cats. Pneumonia is not uncommon and most cats recover. Classically described histologic lesions include hyaline membranes and neutrophilic and serofibrinous exudate in alveoli, not seen in this case. Additional gross findings including mucosal vesicles and ulceration were not seen in this case(1). The vaccination status in this case is unclear, and the low CT value in this case could represent vaccine exposure rather than natural infection.
Bronchiectasis in cats is usually attributed to chronic bronchitis, bronchopneumonia, or pulmonary neoplasia(2). A case series in 2000 reported chronic bronchitis and bronchiolitis, neoplasia, bronchopneumonia, endogenous lipid pneumonia and emphysema as the etiology in 12 adult cats(7). The age of this cat could suggest a congenital ciliary dyskinesia. Diagnostic modality to examine ciliary function or ultrastructure are not routinely available as part of our diagnostic workup. In this case, the functional obstruction due to marked inflammatory infiltrate was interpreted as sufficient to explain the bronchiectasis.
Absent vascular or gastrointestinal lesions, the positive PCR finding for feline coronavirus is interpreted clinically insignificant.
Although this kitten was the only one of three kittens in the litter affected, a second kitten born to the same dam in a subsequent litter presented with similar clinical signs and gross pathology. In addition to similar lung findings, this 7 week old male kitten presented a mediastinal, purulent abscess. Histology, microbiology and viral PCR on the second kitten are pending at the time of press.
JPC Diagnosis:
1. Lung: Pneumonia, bronchointerstitial and proliferative, neutrophilic and histiocytic, diffuse, marked with neutrophilic and lymphoplasmacytic bronchitis, bronchiolitis, bronchiectasis, and lymphoid hyperplasia.
2. Lymph node: Reactive hyperplasia, diffuse, moderate.
Conference Comment:
Mycoplasma cell membranes contain superantigens which bind multiple T-cell receptors (TCR) and non-specifically stimulate the activation of large numbers of T-cells, resulting in lymphoid hyperplasia of the bronchiolar-associated lymphoid tissue (BALT), as is seen in this case. The subsequent massive immune response, which is not specific to any epitope, undermines the ability of the adaptive immune system to target antigens with a high degree of specificity. There is also a massive release of cytokines and resultant inflammatory response, resulting in further tissue damage(8).
The moderator discussed how bronchiectasis, likely caused by inflammatory obstruction, predisposes the lung to secondary infections. Ectatic airways result in decreased airflow which allows the settling of particulates and the deposition of microorganisms. Subsequent inflammation and hypoxia further exacerbates the bronchiectasis, continuing the cycle.
Conference participants also discussed the histologic difference between active bronchiolitis and the presence of a retrograde alveolar exudate. Active bronchiolitis is the observance of active inflammation at the level of the bronchiole, as well as corroborating evidence such as epithelial proliferation, inflammation in the adjacent interstium, and leukocyte transmigration. A retrograde alveolar exudate is simply the presence of an exudate within the bronchiole, likely an extension of an alveolar exudate, without any corroborating evidence of bronchiolar disease.
Some slides included sections of tracheobronchial lymph node with lymphocytolytic debris, likely caused either by stress or viral infection.
References:
2. J Caswell and K Williams: Bronchiectasis. In: Pathology of Domestic Animals, ed. M Maxie, 5th ed., vol 2, pp. 557-9. Elsevier Saunders, Philadelphia, PA, 2007
3. J Chandler, M Lappin: Mycoplasmal Respiratory Infections in Small Animals: 17 Cases (1988-1999): Journal of the American Animal Hospital Association 38: 111-119, March/April 2002
4. S Foster, V Barr, P Martin, R Malik: Pneumonia associated with Mycoplasma spp in three cats: Aust Vet J Vol 76 (7): 460-4, 1998
5. S Foster, P Martin, G Allan, V Barrs, R. Malik: Lower respiratory tract infections in cats: 21 cases (1995 2000): Journal of Feline Medicine and Surgery 6: 167180, 2004
6. Hussain and V Kumar: The Lung. In: Pathologic Basis of Disease 7th edition, ed. Kumar, Abbas, Fausto ed., Elsevier Saunders, Philadelphia, PA, 2005
7. C Norris, V Samii: Clinical, radiographic, and pathologic features of bronchiectasis in cats: 12 cases (19871999). JAVMA 216(4): 530-534, 2000
8. Stricker TP, Kumar V. Neoplasia. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds., Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:132.
9. AV Trow, EA Rozanski, AS Tidwell: Primary mycoplasma pneumonia associated with reversible respiratory failure in a cat. Journal of Feline Medicine and Surgery 10: 398-402, 2008