Signalment:
Gross Description:
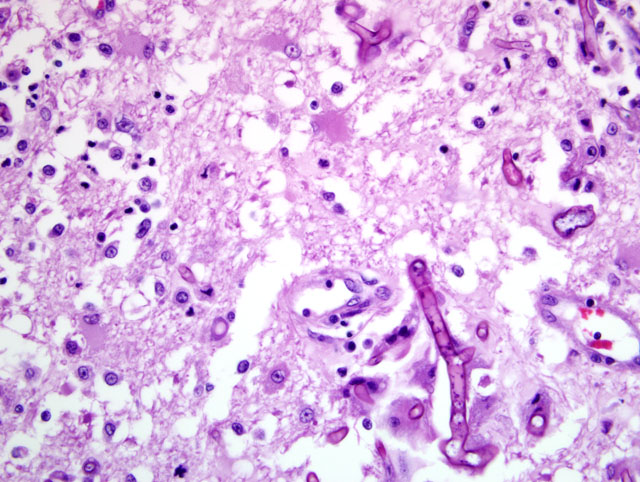
Histopathologic Description:
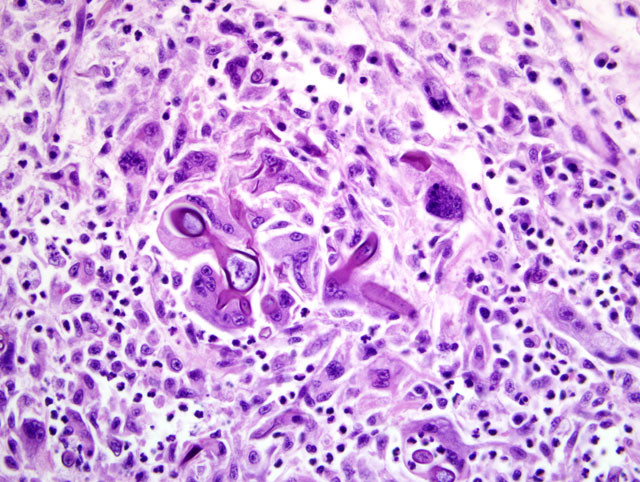
Heart: A section of the heart reveals multifocal to coalescing nodules within the myocardium composed of fungal hyphae and inflammatory populations similar to those described above. Inflammatory populations include numerous histiocytes and neutrophils with fewer multinucleated giant cells, and lymphocytes and plasma cells on a background of loose connective tissue stroma. Histiocytes multifocally contain abundant, granular, brown cytoplasmic pigment (hemosiderin). Entrapped myofibers are often hypereosinophilic and shrunken with loss of cross striations, rarely vacuolated sarcoplasm and pyknotic nuclei (degenerative change). There is mild interstitial edema and few Anitschkow cells characterized by elongated, undulating nuclei. Multifocally, there are few foci of interstitial fibrosis in the remaining, unaffected myocardium.Â
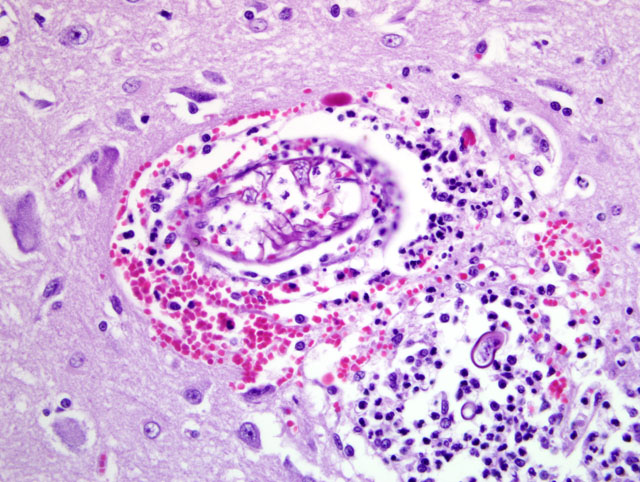
Tracheobronchial lymph node (tissue not included): The tracheobronchial lymph node is diffusely effaced by pyogranulomatous inflammation containing fungal hyphae similar to those described above. This inflammation is often partially surrounded by fibrous connective tissue. Inflammatory cells extend to the adjacent pulmonary parenchyma and vessels, which are partially to completely occluded by the granulomas (Fig 5). Vascular changes, including fibrinoid degeneration and inflammation similar to that described in the brain, is also multifocally present.Â
Morphologic Diagnosis:
1. Brain, cerebral cortex: Severe, multifocal to coalescing, chronic ongoing pyogranulomatous encephalitis with intralesional fungal hyphae and multifocal fibrinonecrotizing vasculitis.Â
2. Heart: Severe, multifocal to coalescing, chronic ongoing, pyogranulomatous myocarditis with intralesional fungal hyphae, myocardiocyte degeneration and loss.Â
3. Tracheobronchial lymph node (tissue not included): Severe, diffuse, chronic-ongoing pyogranulomatous lymphadenitis with invasion into adjacent pulmonary parenchyma, multifocal fibrinonecrotizing vasculitis and intralesional fungal hyphae.
Lab Results:
Condition:
Contributor Comment:
Alternaria alternata is a dematiaceous (pigmented), saprophytic fungus that is ubiquitous in the environment (indoors and outdoors) and can be isolated from the skin or fur of healthy humans and animals.(4,13) It is an uncommon cause of phaeohyphomycosis in humans with reports of cases in cats, dogs, goats, horses and deer.(4) In humans, lesions are mainly cutaneous with cases of osteomyelitis, sinusitis, keratitis, onychomycosis and pulmonary granulomas.(3,4,13) In veterinary species, lesions are usually confined to the skin and subcutis with few reports of nasal involvement in cats and keratitis in a horse.(4,5,9,11) Human cases are associated with hypersensitivity syndromes such as woodworkers lung, asthma and eosinophilic pneumonia.(9,11,13) Because of the ubiquitous nature of the fungus, immunosuppression is often suspected, if not proven, in clinical cases.(11-13)
Phaeohyphomycoses are usually opportunistic infections in which fungi generally gain entry via skin wounds.(5,7). Systemic or visceral phaeohyphomycoses are rare, but seem to have a predisposition for the central nervous system (CNS).(1,3,6,10) In these cases, the route of infection is thought to be inhalation with subsequent hematogenous spread to the brain.(3,6,10) CNS phaeohyphomycoses are almost always attributed to Cladophialophora bantiana (previously known as Torula bantiana, Cladosporium bantianum, Xylohypha bantiana, Cladosporium trichoides and Xylohypha emmonsii).(1,10) This species exhibits marked neurotropism, which is related to the presence of introns at the 18S rDNA subunit.(1). There is a single report of fatal systemic illness in a cat due to Cladophialophora bantiana without CNS involvement.(6) Other species implicated in phaeohyphomycoses in cats include A. infectoria, Exophiala jeanselmai, Dreschlera spicifera, Exophiala spinifer, Fonsecaea pedrosoi, Moniliella suaveolens, Scolecobasidium humicola, Staphylotrichum coccosporum, and Ochronic gallopavum.(1,11,12)
Systemic illness in cats due to Alternaria species has not been reported. It is interesting that this cat also had CNS involvement, although the clinical progression suggests a tracheobronchial lymphadenitis that disseminated to the brain rather than primary brain lesion. No cutaneous lesions were noted in this case, and it is suspected that inhalation may be the route of infection. Alternaria spp. have small spores that may allow it to gain entry to lower airways and subsequently to draining lymph nodes.(5)
Identification of dematiaceous fungi requires culture and examination of fungal morphology or molecular techniques such as PCR. Alternaria spp. in culture are pigmented, with tapering of one end of the conidia to form a characteristic beak. The shape of the beak can help differentiate between different species. The conidia form long chains, which may or may not branch depending on the species.(13)
Dematiaceous fungi always form pigment in culture; however, pigment may not be readily visible in H&E tissue sections.(3) Often, melanin stains, such as Fontana-Masson, are recommended to highlight the presence of melanin in hyphae.(3,5,12) In this case, Fontana-Masson was performed on the tissue sections from the affected cat, as well as in a control case of non-pigmented fungus (morphology consistent with Aspergillus sp.), and revealed staining of the hyphae of both types of fungi. Fontana-Masson staining of fungal hyphae should be interpreted with caution, as there may be nonspecific uptake of silver stain in fungal hyphae as demonstrated in this case. A copper-sulfide-silver method (2) described for fungal cultures was modified for use in fixed tissue to try to find a more discriminatory staining technique. This method resulted in inconsistent staining of all fungal hyphae examined (presumed melanized as well as non-melanized).
JPC Diagnosis:
1. Brain, cerebrum: Encephalitis, necrotizing and pyogranulomatous, multifocal to coalescing, severe, with vasculitis and fungal hyphae.Â
2. Heart: Myocarditis, pyogranulomatous, multifocal to coalescing, marked, with myocardial degeneration, necrosis and loss, and fungal hyphae.Â
Conference Comment:
The contributor provided an excellent review of phaeohyphomycosis in general, and alternariosis in particular. Most conference participants considered disseminated zygomycosis to be the most likely etiologic diagnosis, which is not surprising in light of the lack of pigmentation in this particular case. Zygomycosis refers to infection by fungi of the orders Entomophthorales (Basidiobolus sp. and Conidiobolus sp.) and Mucorales (Mucor sp., Rhizopus sp., Rhizomucor sp., and Absidia sp.). In both, hyphae are broad and poorly septate; however, in entomophthoromycosis they are characteristically bounded by brightly eosinophilic sleeves, which are lacking in this case.(8) For many participants, the differential diagnosis also included hyalohyphomycosis caused by nondematiaceous opportunistic fungi that also form hyphae in tissue. These differ from the hyphae in phaeohyphomycosis in that they generally have thinner walls and are not pigmented.(8) This case highlights the utility of molecular diagnostics in practice.
The incidence of phaeohyphomycosis is increasing, resulting in a dramatic increase in importance in human medicine in recent years.(13) As noted by the contributor, it is important to recall that while the production of pigment is characteristic of dematiaceous fungi in culture, it may not be visible on H&E sections. Alternaria infectoria is the most common clinically important species in human cases of alternariosis, but its lack of pigmentation and poor ability to sporulate in routine media make it difficult to diagnose. As a result, numerous cases have been erroneously attributed to A. alternata or A. tenuissima, when A. infectoria was the true etiology.(13)
In contrast to localized phaeohyphomycosis, which is only rarely associated with immunosuppression in dogs and cats, disseminated disease is usually associated with immunosuppression. The polyclonal gammopathy present in this case is common with this disease, indicating a persistent, but ineffective humoral immune response.(8)
References:
2. Butler MJ, Gardiner RB, Day AW: Fungal melanin detection by the use of copper sulfide-silver. Mycologia 97(2):312-319, 2005
3. Chandler FW, Kaplan W, Ajello L: Color Atlas and Text of the Histopathology of Mycotic Diseases, pp. 10, 92-95. Year Book Medical Publishers, Inc. Chicago, IL, 1980
4. Dhein CR, Leathers CW, Padhye AA, Ajello L: Phaeohyphomycosis caused by Alternaria alternata in a cat. JAVMA 193:1101-1103, 1988
5. Dye C, Johnson EM, Gruffyd-Jones TJ: Short communication: Alternaria species infection in nine domestic cats. J Fel Med Surg 11:332-336, 2009
6. Elies L, Balandraud V, Boulouha L, Crespeau F, Guillot J: Fatal systemic phaeohyphomycosis in a cat due to Cladophialophora bantiana. J Vet Med 50:50-53, 2003
7. Genovese LM, Whitbread TJ, Campbell CK: Cutaneous nodular phaeohyphomycosis in five horses associated with Alternaria alternata infection. Vet Rec 148:55-56, 2001
8. Grooters AM, Foil CS: Miscellaneous fungal diseases. In: Infectious Diseases of the Dog and Cat, ed. Greene CE, 3rd ed., pp. 637-650. Saunders Elsevier, St. Louis, MO, 2006
9. Kalina WV, Anderson ML, Gershwin LJ: Alternaria aerosol during a bovine respiratory syncytial virus infection alters the severity of subsequent re-infection and enhances IgE production. Comp Immuno Microbio & Infectious Diseases 29:138-156, 2006
10. Mariani CL, Platt SR, Scase TJ, Howerth EW, Chrisman CL, Clemmons RM: Cerebral phaeohyphomycosis caused by Cladosporium spp. in two domestic shorthaired cats. JAAHA 38:225-230, 2002
11. McKay JS, Cox CL, Foster AP: Cutaneous alternariosis in a cat. J Small Animal Practice 42:75-78, 2001
12. Outerbridge CA, Myers SL, Summerbell RC: Phaeohyphomycosis in a cat. Can Vet J 36:629-630, 1995
13. Pastor FJ, Guarro J: Alternaria infections: laboratory diagnosis and relevant clinical features. Clin Microbiol Infect 14:734-746, 2008