CASE 2: Case 2 (4153150-00)
Signalment:
8-week-old, male entire, domestic short hair cat (Felis catus)
History:
This kitten was found on the side of the road and was presented to the Massey University Pet Emergency Centre by a member of the public. On arrival to the clinic, the kitten was hypothermic and hypoglycemic. Following administration of IV fluids and dextrose, the kitten recovered and was sent to the SPCA.
A week later, the kitten re-presented to the clinic in lateral recumbency with bradycardia, evidence of severe diarrhea (perineal staining) and agonal gasping. Due to poor prognosis, euthanasia was elected.
Gross Pathology:
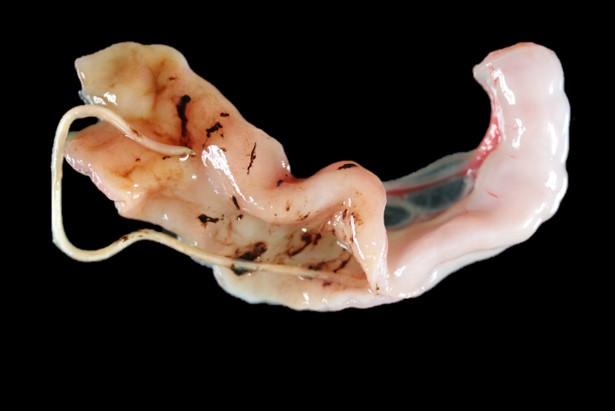
The kitten was in an emaciated state of nutrition (with a body condition score of 1/9 and a total body weight of 0.514 kg) and was markedly dehydrated with markedly prolonged skin tenting and marked enophthalmos. Significant fecal staining around the perineum, under the tail and on the hindlimbs was present.
On internal examination, the stomach was empty except for red-brown mucus over the pyloric mucosa. The intestines were mostly empty except for scant amounts of reddish-black granular material (digested blood) loosely adhered to the intestinal mucosa. There were also 5 white nematode parasites ranging between 3 and 5 cm long in the small intestine (T. cati). The colon contained scant, unformed reddish-brown feces. No other gross lesions present else.
Laboratory results:
None.
Microscopic description:
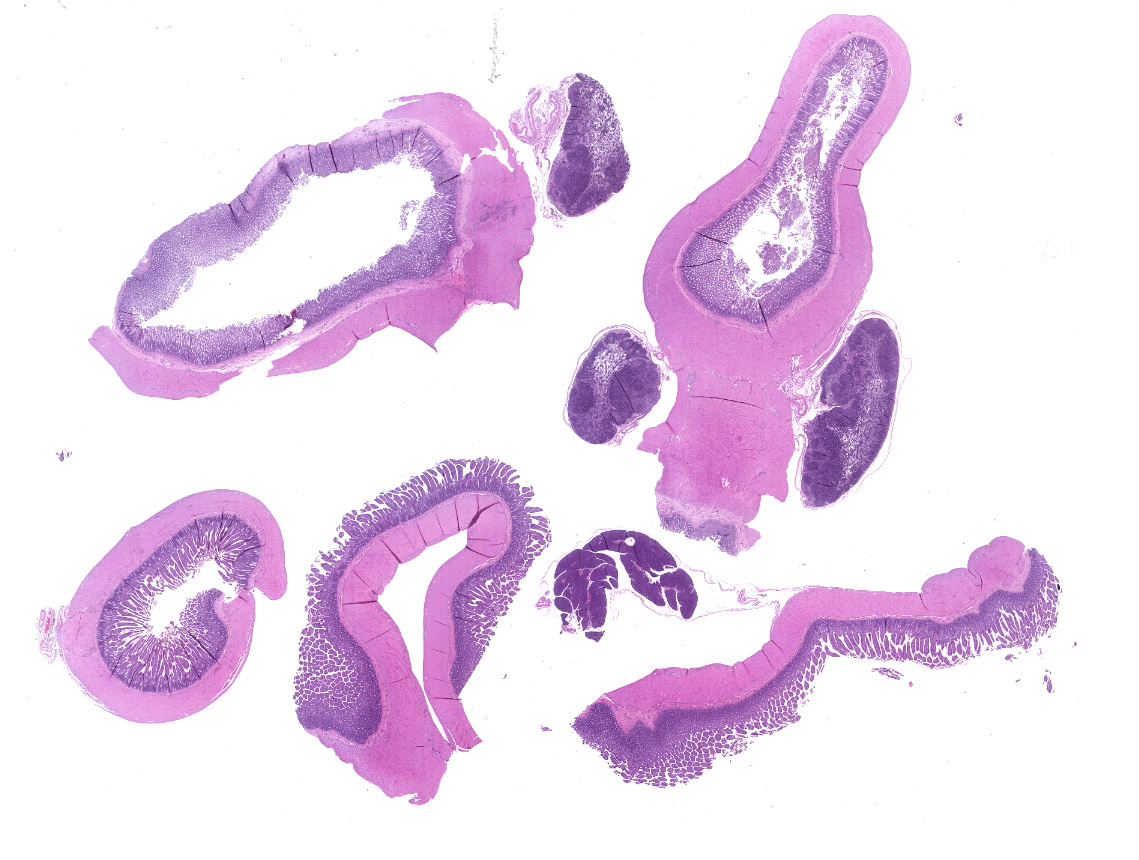
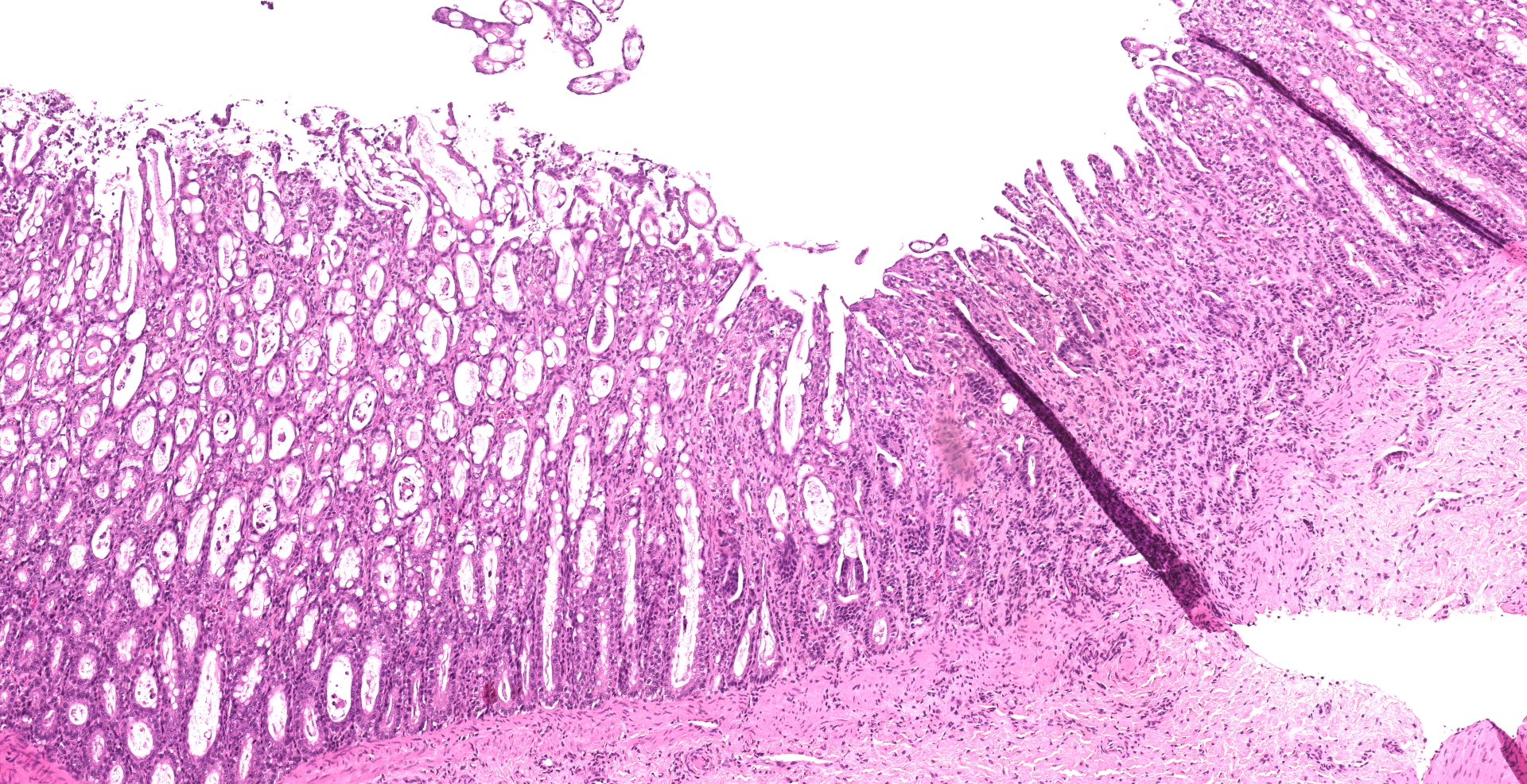
Small intestine: Villi and crypts are segmentally effaced and replaced by extensive infiltrates of neutrophils, large colonies of small uniform Gram-negative bacilli, fibrin, hemorrhage and cellular debris. Remaining crypts within these are areas are ectatic, lined by cuboidal to attenuated epithelial cells, and contain neutrophils, sloughed enterocytes and cellular debris within luminal spaces. Other crypts contain hyperplastic, piling epithelium with increased numbers of mitotic figures (regeneration).
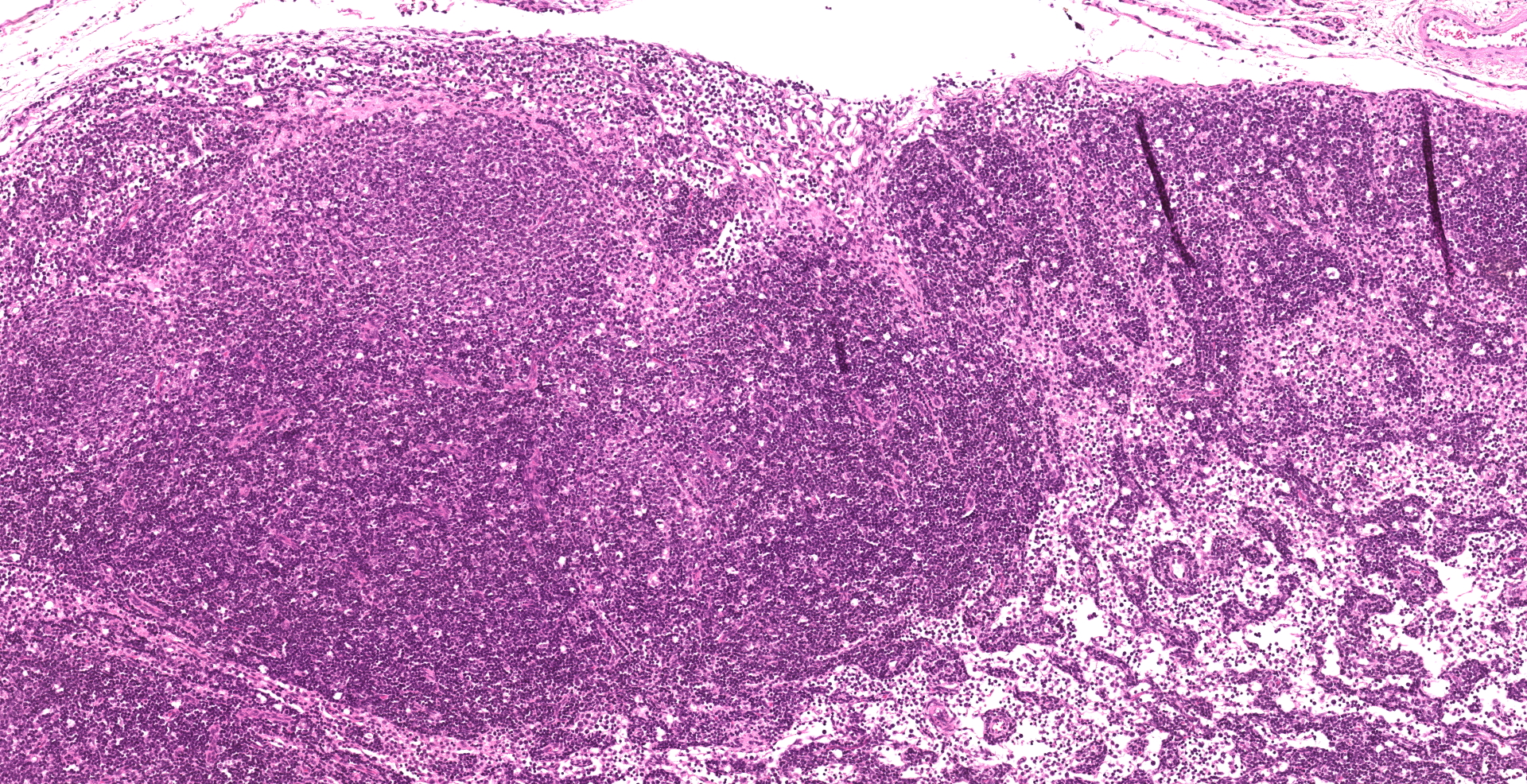
Enterocytes within the crypts multifocally have marginated nuclear chromatin and bright eosinophilic intra-nuclear inclusions (viral inclusions). Adjacent mucosal villi are mildly to moderately blunted and fused with increased numbers of neutrophils and macrophages expanding the lamina propria. A focal area of the submucosa is infiltrated by large numbers of degenerate neutrophils and Gram-negative bacilli. Peyer's patches show marked lymphoid depletion with the presence of lymphocytolysis and large numbers of tingible body macrophages within follicular centers.
In one section of jejunal lumen is a 1 mm x 2mm cross-section of a nematode larvae with coelomyarian musculature, a ridged cuticle, intestinal tract, uteri containing approximately 15 -20 eggs each and ovarian structures.
Contributor's morphologic diagnosis:
Small intestine: Severe, acute segmental necrosuppurative enteritis with crypt necrosis, intra-nuclear viral inclusions, Gram-negative bacilli and nematode larvae
Contributor's comment:
The intestinal lesions in this case are strongly suggestive of feline panleukopenia and secondary bacterial enteritis which were likely significant contributors to the poor body condition and diarrhea in this kitten. Small numbers of Toxocara cati were also present in the small intestine, which may have further contributed to the emaciation and diarrhea but were not causing obstruction of the bowel and were unlikely to be the leading cause of death.
Feline panleukopenia virus (FPV) is a member of the feline parvovirus subgroup within the Parvovirus genus (family Parvoviridae) and is closely related to mink enteritis virus (MEV) and canine parvovirus-2 (CPV-2).3 FPV is a relatively small (25nm) non-enveloped single-stranded DNA virus with a genome of approximately 5000 bases.12 Due to the highly stable nature of FPV within the environment, the large quantities of virions shed in feces (107-109 per gram), and the ability of the virus to remain infectious for weeks post-shedding, FPV is considered an ubiquitous pathogen found in almost all regions of the world.8
Although FPV has been proven to affect cats of all ages, kittens tend to be most susceptible to the disease.11 The predisposition of kittens contracting the disease is due to kittens experiencing a precarious period of sub-optimal immunity against the virus between 8-12 weeks of age where levels of circulating maternally derived antibodies (MDA) are insufficient to protect against infection yet high enough to interfere with vaccination.11 Despite most cats not developing clinical disease following infection, those who do develop overt disease are at great risk of death with mortality rates of ? 90% reported in the literature.11
The most common clinical signs observed in affected cats include anorexia, diarrhea, vomiting, lethargy and pyrexia.7 Co-infection of the host with intestinal parasites (such as T. cati in this case), bacteria, or other viruses (e.g. rotaviruses or coronaviruses) are likely to increase the severity of the disease.8 Infected cats tend to die from complications associated with translocation of bacteria into the bloodstream, sepsis and disseminated intravascular coagulopathy (DIC).5 The most common laboratory abnormalities present in affected cats include leukopenia, thrombocytopenia, anemia, neutropenia, hypoalbuminemia and lymphopenia.7 It is important to note that kittens infected late during their development in utero exhibit a distinct manifestation of the disease characterized by nervous signs (namely ataxia and hypermetric gait) due to viral induced cerebellar hypoplasia.13
The route of entry of FPV into the host is oronasal with subsequent uptake of the virus into the epithelium overlying the tonsils and/or Peyer's patches.13 Following host inoculation, lymphogenous spread of the virus may result in infection of other lymphatic structures including the thymus, spleen and lymph nodes. Virally induced lymphocytolysis within these lymphatic structures induces cell-free hematogenous spread of the virus to a variety of different organs throughout the body. It is late during this viremic period (approximately 5-7 days post infection) in which most clinical signs are observed.13
As FPV requires the 'hijacking' of host cell DNA polymerases and other DNA replication machinery, the virus has a tropism for mitotically active cells within the host.12 The predominant cells infected by FPV outside of lymphoid tissue are enterocytes in crypts of Lieberkühn adjacent to or near Peyer's patches and stem cells within the bone marrow.12 Invasion of FPV into these mitotically active host cells is achieved through endocytosis mediated by viral capsid proteins binding to host neuraminic acid and transferrin receptors on the cell surface.13 Once within the host cell, the virus is only able to replicate during the S-phase of the DNA replication cycle. Following viral replication, FPV virions are released from infected cells with subsequent lysis and death of the host cell.
In the case of infected enterocytes, lysis of these cells results in the release of virions into the intestinal lumen thus contaminating the feces. Destruction of enterocytes results in a markedly reduced absorptive surface within the small intestine and subsequent diarrhea. It should also be noted that effusion of tissue fluids and blood from denuded intestinal mucosa into the lumen is also hypothesized as a contributing cause of diarrhea in FPV infected patients.13 Anemia and hypoproteinemia are also hypothesized to result from effusion of tissue fluids and blood from denuded intestinal mucosa into the lumen. The hallmark panleukopenia observed in individuals infected by FPV is the result of both lymphocytolysis within lymphoid tissues and destruction of myeloid precursors in bone marrow. Histopathologically, lysis of stem cells within the bone marrow is appreciated as marked inter-trabecular hypocellularity.13
Additional confirmatory diagnostic tests for FPV include immunohistochemistry of affected tissue, fecal ELISA, fecal PCR, fecal latex agglutination test (LAT) or fecal immunochromatography.11,13
Contributing Institution:
Massey University
School
of Veterinary Science
New Zealand
JPC diagnosis:
1. Small intestine: Enteritis, necrotizing, diffuse, severe, with villar blunting, crypt hyperplasia, crypt abscesses, lymphoid depletion, and rare intranuclear viral inclusions.
2. Lymph node: Lymphoid depletion, diffuse marked.
JPC comment:
A brief discussion was held regarding positively charged glass slides, and how important these are for tissue retention during processing. Positively charged slides are marked with a "+" and are purchased with this feature. A number of tissues have been lost during processing of immunohistochemical stains, and this feature greatly increases the likelihood of not losing the tissue.
The contributor provides a broad overview of feline panleukopenia and many of the presentations of this disease. Feline panleukopenia is the oldest known viral disease of cats and is a well-known pathogen around the world. Carnivore protoparvovirus 1 is the base virus previously described, which contains two open reading frames (ORFs). ORF-L codes for two non-structural proteins, NS1 and NS2; ORF-R codes for two structural proteins, VP-1 and VP-2 (major capsid protein). Feline parvovirus, canine parvovirus, mink enteritis virus, and others are different strains of carnivore protoparvovirus 1, but there is a high degree of homology between the sequences of their capsid proteins.1,2 Binding of the transferrin receptor (TfR) is crucial for productive infection, which then allows for the virus to be taken into the host cell through clathrin-mediated endocytosis.1
While closely related, canine parvovirus 2 (CPV-2) differs from feline panleukopenia virus by 6 amino acids in the VP-2 region, preventing efficient binding of canine TfR and clinical disease does not develop. However, there are a number of variants of CPV-2 that are capable of binding both canine and feline TfRs and leading to disease in both species (CPV-2a, CPV-2b, CPV-2c).1
In recent research, an additional histologic finding has been cytoplasmic vacuolation of neurons in the thoracic spinal cord of an infected cat. Using advanced sequencing techniques, feline panleukopenia virus antigen was recovered from vacuoles, as well as neuronal cytoplasm, suggesting that neuronal vacuolation is a histologic change consistent with feline panleukopenia virus infection.9
There have been confirmed cases of feline panleukopenia virus causing disease in other species. There is a high degree of homology between mink enteritis viruses (MEV-1, MEV-2, MEV-3) and feline panleukopenia virus. A recent phylogenetic analysis of viruses isolated from a mink farm suggested that several strains of MEV would more appropriately be considered FPV, instead.4 More recently in Thailand, a banded linsang presented with signs consistent with parvoviral enteritis, and tested PCR positive for CPV. However, upon sequencing the VP2 gene of the virus, it was determined to be FPV, similar to strains reported in Japan and South Korea.6 These overlapping test results highlight the similarity of these viruses.
In another disease, a parvovirus has been recently implicated as the most plausible etiologic agent. Theiler's disease in horses, also known as equine serum hepatitis, is characterized by an acute, massive, diffuse hepatic necrosis that is often life-threatening. A number of agents have been implicated, and it appeared to have a correlation with equine serum derived biologics, including tetanus antitoxin, botulinum antitoxin, Streptococcus equi antiserum, pregnant mare's serum, and equine plasma. Two flaviviruses were suggested as the cause and included non-primate hepacivirus (NPHC) and Theiler's disease-associated virus (TDAV). However, additional analysis has shown that a startling number of biologics have been PCR positive for Equine parvovirus-hepatitis (EqPV-H). While there were other positives for NPHV, TDAV, and Equine pegivirus (EPgV), all samples of affected horses were PCR positive for EqPV-H.10
References:
- Barrs VR. Feline Panleukopenia, a Re-emergent Disease. Vet Clin Small Anim. 2019;49(4):651-670.
- Cheng N, Zhao Y, Han Q, et al. Development of a reverse genetics system for a feline panleukopenia virus. Virus Genes. 2019;55(1):95-103.
- Decaro N, Buonavoglia D, Desario C, et al. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia. Res. Vet. Sci. 2010;89:275-278.
- Fei-fei D, Yong-feng Z, Jian-li W. Molecular characterization of feline panleukopenia virus isolated from mink and its pathogenesis in mink. Veterinary Microbiology. 2017;205:92-98.
- Hartmann K. Feline panleukopenia ? update on prevention and treatment. Thai J Vet Med Suppl. 2017;47:101-104.
- Inthong N, Sutacha K, Kaewmongkol S, et al. Feline panleukopenia virus as the cause of diarrhea in a banded linsang (Prionodon linsang) in Thailand. J Vet Med Sci. 2019;81(12):1763-1768.
- Kruse BD, Unterer S, Horlacher K, Sauter-Louis C, Hartmann. Prognostic Factors in Cats with Feline Panleukopenia. J Vet Intern Med. 2010;24:1271?1276.
- Parrish CR. Pathogenesis of feline panleukopenia virus and canine parvovirus. In: Kerr JR, Cortmore SF, Bloom ME, Linden RM, Parrish CR, ed. Parvoviruses. 5th ed. London, UK: Hodder Arnold; 2006:429-434.
- Pfankuche VM, Jo WK, van der Vries E, et al. Neuronal vacuolization in feline panleukopenia virus infection. Veterinary Pathology. 2017;55(2):294-297.
- Tomlinson JE, Kapoor A, Kumar A, et al. Viral testing of 18 consecutive cases of equine serum hepatitis: A prospective study (2014-2018). Journal of Veterinary Internal Medicine. 2019;33(1):251-257.
- Truyen U, Addie D, Belák S, et al. Feline Panleukpenia: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009;11:538-546.
- Truyen U, Parrish CR. Feline panleukopenia virus: Its interesting evolution and current problems in immunoprophylaxis against a serious pathogen. Vet. Microbiol. 2013;165:29-32.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie GM, Jubb KVF, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. St. Louis, Missouri: Elsevier; 2016;2:153-158.