Signalment:
Gross Description:
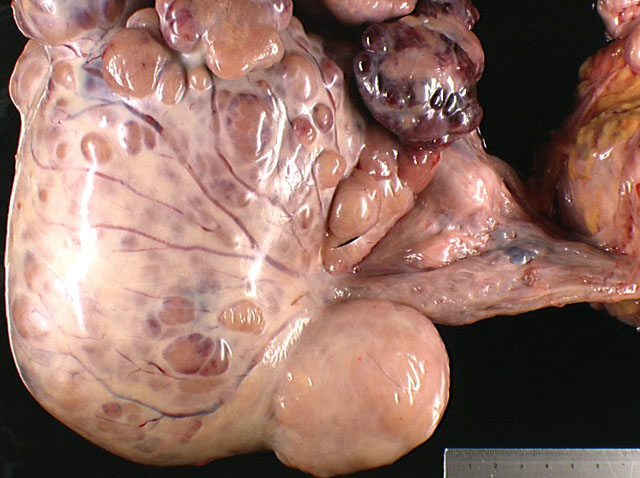
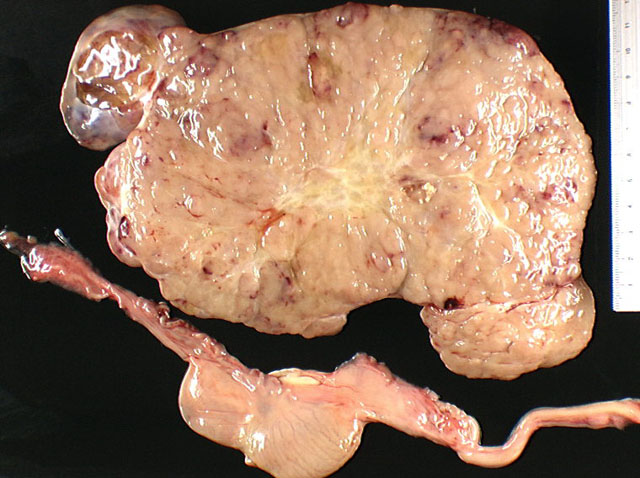
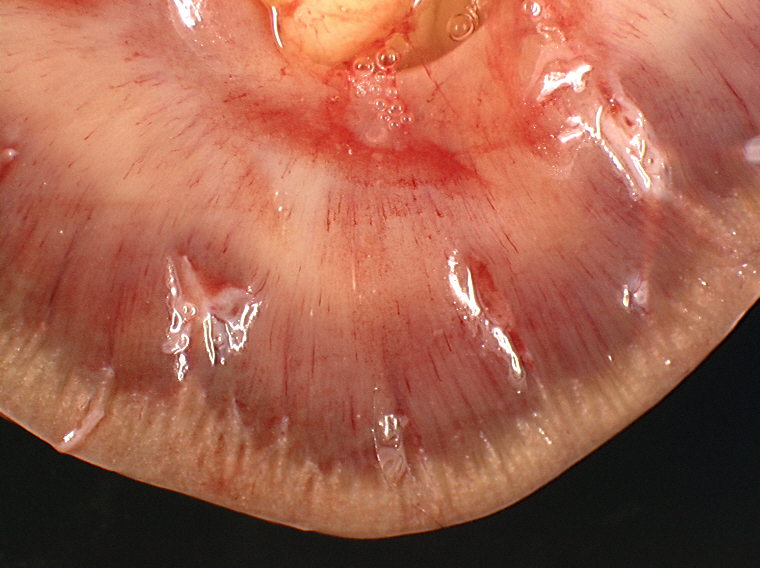
A bilateral cryptorchidism was detected with both testicles being in an intra-abdominal position. The left testicle was severely enlarged (10 x 12 x 20 cm), lobulated, white and moderately firm with foci of haemorrhage and mineralization (Fig.1-1, 1-2). Another mass of similar size and consistency was detected in the left iliac region (most likely left iliac lymph node) around the left ureter and left iliac artery, and vein. Multiple white and moderately firm nodules were attached to the splenic hilus. We made a tentative gross morphologic diagnosis of seminoma with nodal and splenic (most likely by transcoelomic implantation) metastases (Fig. 1-3).Â
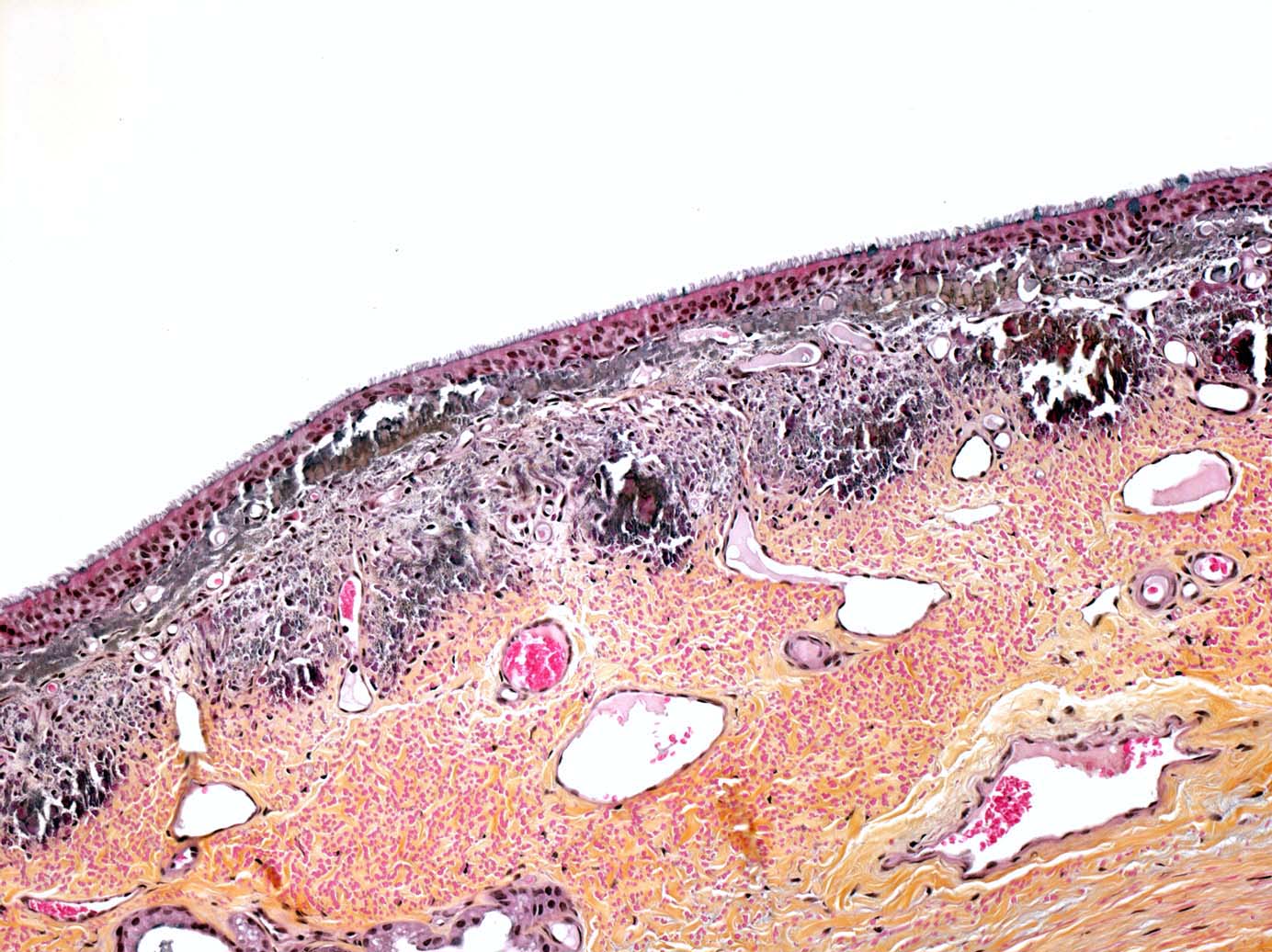
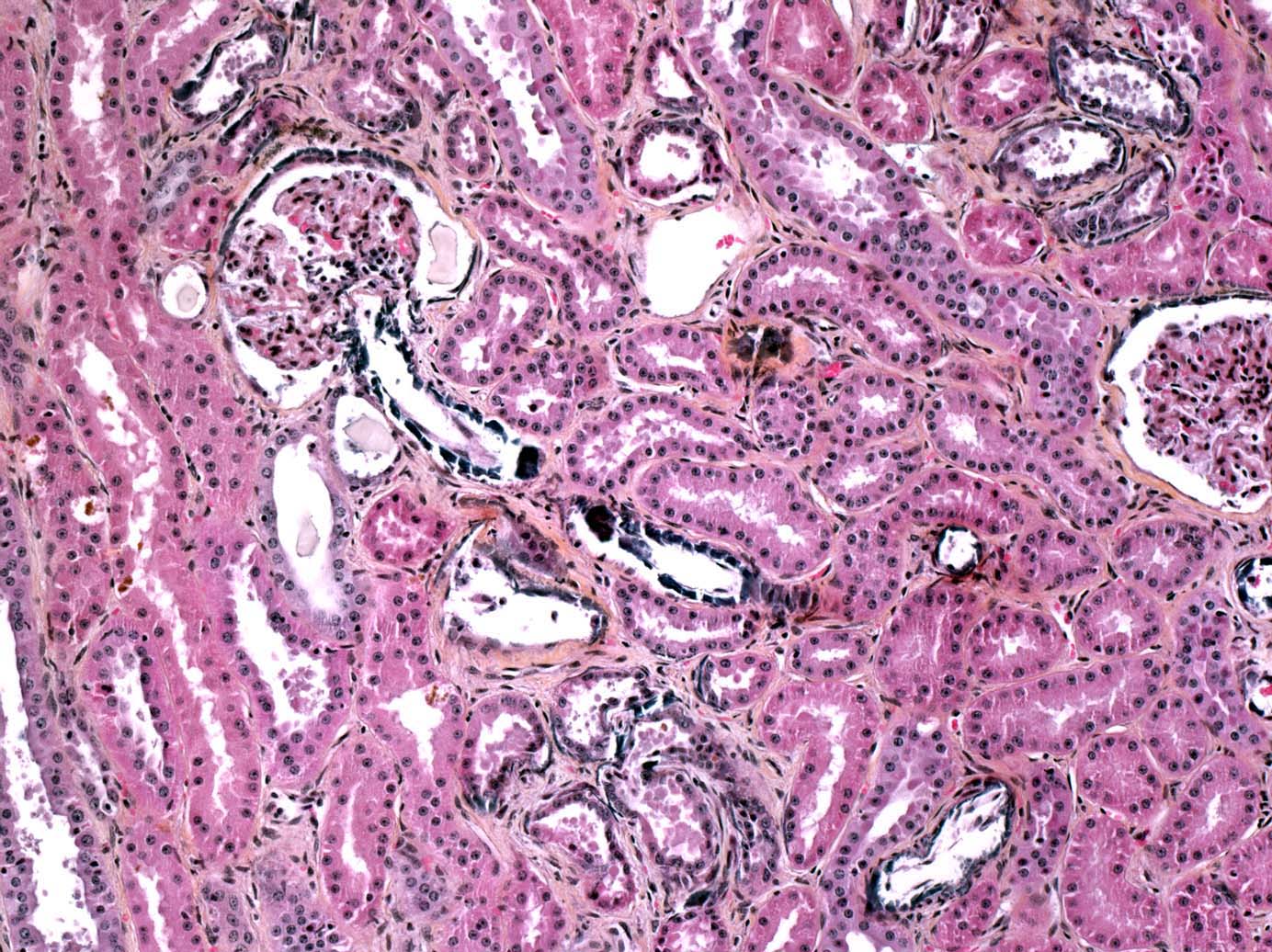
Multifocal endocardial mineralizations, appearing as plaques were observed in the ventricles and atria, particulary in the left side, and in the aortic intima. Mineralization was severe on the sigmoid aortic valves. Multifocal myocardial necrosis and mineralizations were suspected as well. The tracheal mucosa contained small mineralized granules, giving it an appearance of abrasive paper. Mineralizations were also observed in the renal cortex of both kidneys. As parathyroid glands were macroscopically normal, we suspected these lesions to be metastatic calcifications due to humoral hypercalcemia of malignancy (Fig. 1-7, 1-8, 1-9, 1-10) (see Contributors Comment).
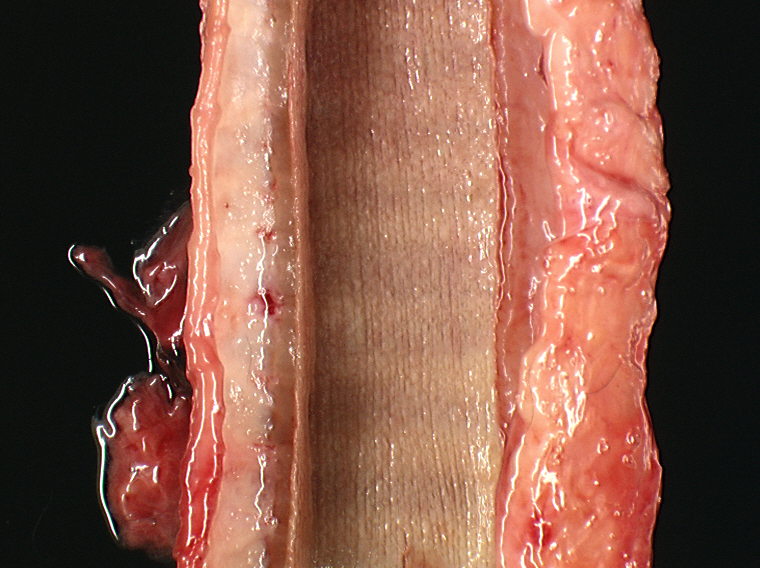
The right testicle was moderately reduced in size (6 x 3 x 3 cm) with a spongy consistency (Fig. 1-2). We made a tentative gross diagnosis of testicular hypoplasia.
Subcutaneous tissue in caudoventral abdomen and hindlimbs was severely edematous. Because of tumoral involvement of the left iliac nodes and vessels, we hypothesized the acquired lymphedema was secondary to obstruction of lymph flow by the neoplasm.
Histopathologic Description:
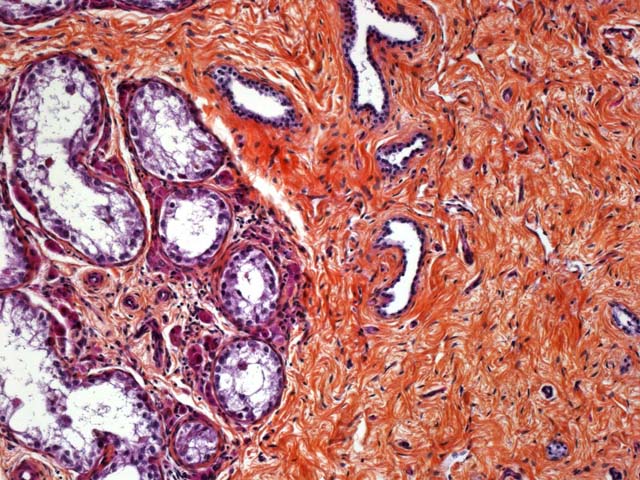
Spleen: The peritoneal side of the splenic capsule is infiltrated by a similar tumoral proliferation (not obvious in all slides). Intimal bodies and diffuse intimal mineralizations are prominent in some arteries.
Right testicle: Testicular tubules are rarefied and separated by large bundles of a dense, mature fibrous connective tissue. They have a markedly diminished number of germinal cells and no spermatozoa with normal to mildly decreased numbers of Sertoli cells, interstitial cells and efferent ductules. Basal membranes of tubules are normal in thickness and are not obviously wrinkled. Some interstitial cells contain brown granular pigments (probably lipofuscin granules).
Morphologic Diagnosis:
2. Splenic capsule: metastasis of seminoma
3. Right testicle: testicular hypolasia
Condition:
Contributor Comment:
Primary testicular tumors may be classified as sex-cord stromal (gonadal stromal) tumors, which include Leydig (or interstitial) cell tumor and Sertoli cell tumor, and germ cell tumors, which include seminoma, embryonal carcinoma and teratoma. Mixed germ cell-sex cord stromal tumors are rare and include gonablastoma. Tumors derived from other testicular elements such as mesothelioma, hemangioma, fibroma and their malignant counterparts are infrequent.Â
Seminomas are common in canine and equine testis. They occur less frequently in the ram, buck and bull. This type of tumor is mostly seen in older animals and is very common in cryptorchid testicles.(2,6) Seminoma is the second most common canine testicular neoplasm and the most common testicular neoplasm in the aged stallion.(2,3,6) Clinically, this type of tumor can be recognised in horses by ultrasonic examination. A diffuse heterogeneous appearance with diffuse hypoechogenicity of the testis and ill-defined nodular regions of hyperechogenicity are characteristic of equine seminomas.(1)
Seminomas develop presumably from basal spermatogonia of the seminiferous tubules. These tumors are seldom malignant and show no hormone production. However, they tend to be locally invasive and there is no known factor to predict their metastatic potential. The actual WHO Classification of Domestic Animals does not recognize a traditional distinction between benign and malignant forms of seminomas, the term seminoma being applied for both. This may reflect in part the influence of human pathology where seminomas are considered malignant (see below). Because of their tendency to be locally invasive with rare metastasis in domestic animals, we should regard them as tumors of generally low malignancy. However, they are more likely to have malignant behaviour than Sertoli and Leydig cell tumors, particularly in dogs and horses.(2,6)
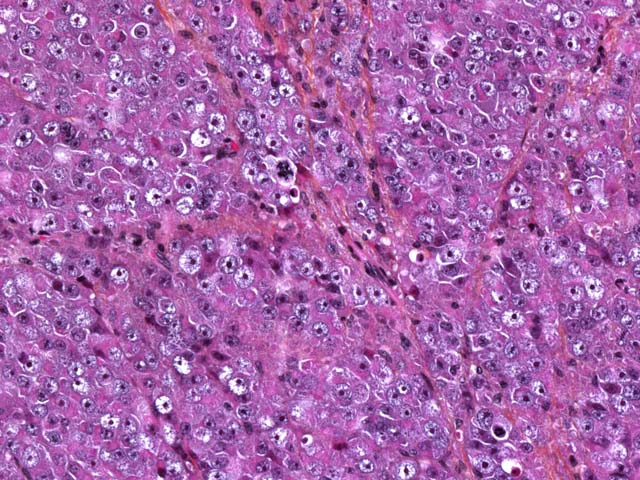
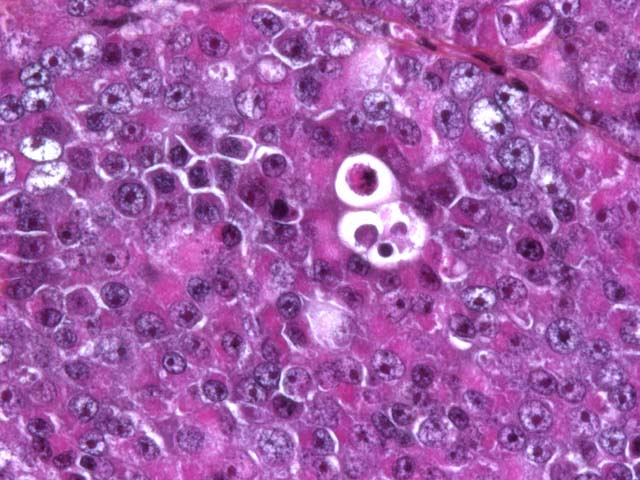
Seminomas are classified on their histological appearance into the intratubular type or the diffuse type. The earliest development of the tumor is intratubular. Rupture of tubules occurs and the growth becomes confluent, forming broad sheets of closely packed cells. Although not prominent in this case, focal or diffuse accumulation of CD8-positive lymphocytes occurs in most seminomas and is a useful diagnostic feature.(2,4,6) Germ cells express vimentin in a perinuclear pattern, but no expression of neuron-specific enolase nor cytokeratin can be detected by immunohistochemistry. Tumoral cells examined by electron microscopy resemble normal germinal epithelium. A relative scarcity of cytoplasmic organelles, oval nuclei, straight cell borders and a distinct Golgi apparatus are characteristic features of seminomas.(2) Differential diagnosis on histological examination includes other round cell tumors, particularly malignant lymphoma.
Macroscopically, involved testicles are enlarged, soft to moderately firm (not as firm as Sertoli cell tumors). On cut surface, the tumor has a homogeneous glistening gray/white appearance, resembling lymphoid neoplasms.(2,6)
In humans, the actual WHO classification of germ cell tumors is more complex:
|
Intratubular germ cell neoplasia, unclassified (IGCNU) (2) Other types Tumors of one histological type (pure forms)
Tumors of more than one histological type (mixed forms) Mixed embryonal carcinoma and teratoma (3) Mixed teratoma and seminoma (3) Choriocarcinoma and teratoma/embryonal carcinoma (3) Others |
(0) = benign; (1) = borderline or uncertain behaviour; (2) = Carcinoma in situ or grade III intraepithelial neoplasia; (3) = malignant
Intratubular germ cell neoplasia, unclassified type (IGCNU) is equivalent to carcinoma in situ or intratubular preinvasive tumor but is different from an intratubular seminoma. It is regarded as a precursor lesion and is associated with cryptorchidism or others conditions. Although potentially present in all species, this entity has not yet been described in domestic animals except, interestingly, in horses.(8)
Cryptorchidism is defined as an incomplete descent of the testis and associated structures into the scrotum and is one of most common abnormalities of the male reproductive system (the most common in cats and horses). Complete testicular descent usually occurs prior to birth in most species, except in dogs. Retained testes lack spermatogenesis and fertility may be compromised. Three main stages are defined during the testicular descent: transabdominal migration phase, intra-inguinal phase and extra-inguinal migration. The regulation of descent involves the M+�-+llerian inhibitory substance in the first phase, increased intra-abdominal pressure in the second phase, interaction of androgen, calcitonin gene-related protein, and other factors in the last stage of migration. Thus, multiple mechanisms can be responsible for cryptorchidism.
An association between testicular neoplasia and cryptorchidism is well recognized in several species, especially in dogs. Dogs with abdominally retained testes are most likely to develop Sertoli cell tumor, Seminoma is the second most common type of tumor of abdominally retained testes in the dog. In stallions, retained testicles are prone to develop into seminoma (most likely) or teratoma. Cryptorchidism also predisposes to testicular torsion in dogs and stallions, particularly if there is tumoral involvement. Cryptorchidism also occurs in boars, bulls and rams.(2,6) Table 1-1 outlines the main characteristics of cryptorchidism in domestic animals. Intratubular germ cell neoplasia, unclassified type (IGCNU) is equivalent to carcinoma in situ or intratubular preinvasive tumor but is different from an intratubular seminoma. It is regarded as a precursor lesion and is associated with cryptorchidism or others conditions. Although potentially present in all species, this entity has not yet been described in domestic animals except, interestingly, in horses.(8)
| Species | Predisposed breeds | Causes | Features |
| Cat | Mostly Persians | Unknown | Mostly unilateral with no side nor site predilection |
| Dog | Various | Autosomal recessive suspected in some breeds | Other diseases associated. Right side and inguinal location mainly |
| Miniature Schnauzer | Persistent M+�-+llerian duct syndrome (M+�-+llerian duct inhibitory substance insensitivity) | Unilateral or bilateral | |
| Boar | Duroc | Hereditary, recessive (several locus may be involved in Durocs) Mainly due to abnormal development of the gubernaculums | |
| Bull | Polled Hereford and Shorthorn | Hereditary | Mostly in inguinal region. Left testis twice as often affected than right. |
| Ram | Polled animals | Autosomal recessive or dominant with incomplete penetrance | Mostly unilateral and involving right testis |
| Buck | Polled Saanen | Goat polled/intersex syndrome | Right testis mostly |
| Stallion | No breed predisposition | Unknown | Mainly unilateral. Left testis mostly abdominal and right testis mostly inguinal |
Testicular hypoplasia is defined as testes that have failed to grow to normal size and is associated with either cryptorchidism, some intersex conditions, or as an isolated lesion. It can be unilateral or bilateral, and the affected testis can be smaller or of similar size compared to the normal testis. Etiology is often multifactorial and can have a hereditary basis. In humans and mice, a deficiency of gonadotrophins (hypogonadotrophic hypogonadism) has been associated with testicular hypoplasia. However, LH and FSH are normal and even elevated in studies concerning domestic animals. Abnormal migration of germ cells to the genital ridge in utero, development arrest, or excessive apoptosis play an important role in some forms of the condition. Histologically, there are reduced numbers, length, and/or diameter of tubules. Germ cells may be present or absent, and if present fail to produce spermatozoa. Basement membranes are not particularly wrinkled nor thickened as in testicular atrophy/degeneration.(2) Table 1-2 outlines the main causes of testicular hypoplasia in domestic animals.
| Species | Causes |
| Cat | Cryptorchid and tricolour/calico cats with XXY genotype (Klinefelters syndrome) |
| Dog | Hereditary in some breeds. Cause of congenital azoospermia. Associated with cryptorchidism, XX reversal, XX syndrome and treatment of the bitch with diethylstilbestrol. |
| Bull | Multifactorial, may be hereditary in Swedish Highland breed as a recessive trait with incomplete penetrance: animals with white body and ears are highly susceptible. Most cases of hypoplasia are unilateral with the left side most often affected. |
| Ram | Sporadic, uni- or bilateral. XXY syndrome reported also. Zinc deficiency implicated in some cases. |
| Buck | Goat polled/intersex syndrome Sporadic forms of unknown cause in other breeds |
In our case, we had the classical association of cryptorchidism with seminoma and testicular hypoplasia. Furthermore, multifocal severe mineralizations were present in the trachea, aorta, renal tubular and glomerular basement membranes (hypercalcemic nephropathy), and endocardium. A moderate hypercalcemia (precise value unknown) was communicated to attending veterinarians by the referring veterinarian. No macroscopic nor microscopic lesions were observed in the parathyroid glands. The ingestion of calcinogenic plants or nutritional imbalances has been excluded by attending clinicians. In such circumstances, the observed mineralizations may reflect a hypercalcemic state, probably induced by the seminoma as a paraneoplastic syndrome (Humoral Hypercalcemia of Malignancy). Interestingly, in human pathology, there are reports of germ cell tumors producing Parathyroid Hormone-related Peptide (PTHrP) with subsequent hypercalcemia.(5,7) To our knowledge, there is no report of paraneoplastic hypercalcemia in connection with germ cell tumors in veterinary medicine. Unfortunately, PTHrP expression could not be investigated in this case.
JPC Diagnosis:
2. Splenic capsule: Seminoma, metastatic
3. Right testicle: Hypoplasia, diffuse, severe
Conference Comment:
| Gross Appearance | Histologic Appearance | |
| Interstitial cell tumor (Leydig cell tumor) |
|
|
| Sertoli cell tumor |
|
|
| Seminoma |
|
|
The three most common testicular neoplasms in the dog are the Sertoli cell tumor, interstitial cell tumor, and seminoma. Grossly and histologically, Sertoli cell tumors have a prominent fibrous component, while the other two tumors have only a small amount of fibrous stroma. Neoplastic Sertoli cells often palisade along tubules and are the only cells of the testes that are immunohistochemically positive for neuron-specific enolase (NSE). The interstitial cell tumor is derived from interstitial endocrine cells. Grossly, the yellow-orange color of the neoplasm reflects the high level of intracytoplasmic yellow lipochrome pigment. Histologically, neoplastic cells are round to polygonal with granular to vacuolated cytoplasm and multifocal hemorrhagic or cystic areas. Seminomas arise from germ cells and grossly appear as white-gray bulging masses. Histologically, seminomas are composed of round cells forming either a diffuse or intratubular pattern with a high mitotic rate.(2)
References:
2. Foster RA, Ladds PW: Male genital system. In: Jubb, Kennedy and Palmer's Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 572-600. Saunders Elsevier, Edinburgh, London, New York, Oxford, Philadelphia, St. Louis, Sydney, Toronto, 2007
3. Galofaro V, Consiglio C, Rapisarda G, Marino F: Bilateral malignant seminoma with metastases in the mule: a report of two cases. Reprod Domest Anim 43:121-123, 2008
4. Grieco V, Rondena M, Romussi S, Stefanello D, Finazzi M: Immunohistochemical characterization of the leucocytic infiltrate associated with canine seminomas. J Comp Pathol 130:278-284, 2004
5. Looijenga LH, Oosterhuis JW: Pathogenesis of testicular germ cell tumours. Rev Reprod 4:90-100, 1999
6. MacLachlan NJ, Kennedy PC: Tumors of the genital systems. In: Tumors in Domestic Animals, ed. Meuten DJ, 4th ed.pp. 561-567. Iowa State Press, Ames, IA, 2002
7. Sorscher S: Elevated parathyroid hormone-related peptide in a patient with an extragonadal germ-cell tumour and hypercalcemia. Can J Surg 47:144, 2004
8. Veeramachaneni DN, Sawyer HR: Carcinoma in situ and seminoma in equine testis. Apmis 106:183-185; discussion185 -186, 1998
9. Woodward PJ, Heindenreich A, Looijenga LH, Oosterhuis JW, McLeod DG, Moller H, Manivel JC, Mostofi FK, Hailermariam S, Parkinson MC, Grigor K, True L, Jacobsen GK, Oliver TD, Talerman A, Kaplan GW, Ulbright TM, Sesterhenn IA, Rushton HG, Michael H, Reuter VE: Germ cell tumours. In: World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, eds. Eble JN, Sauter G, Epstein JI Sesterhenn IA, pp. 221-249. IARC Press, Lyon, 2004