Signalment:
Gross Description:
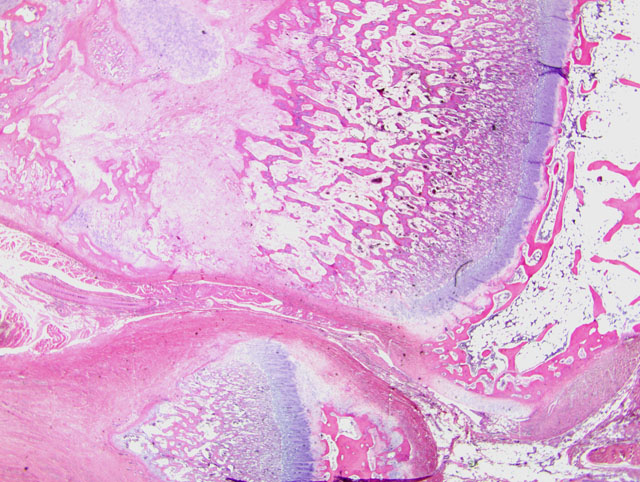
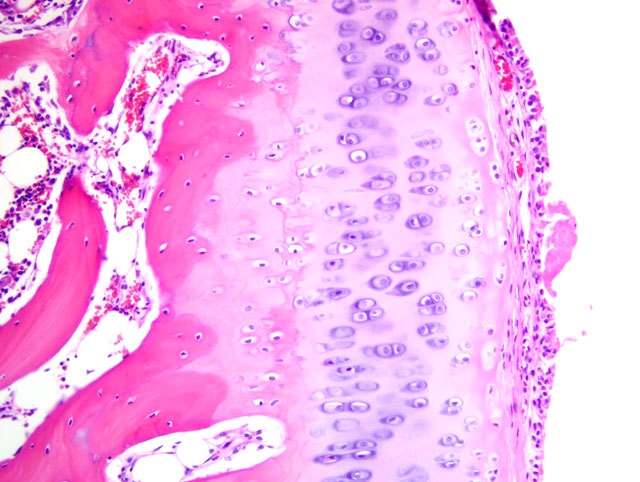
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Osteogenesis imperfecta is an inherited disease caused by a genetic defect of the COL1A1 or COL1A2 genes, which encode the procollagen molecules of collagen type 1. This results in quantitative and qualitative abnormalities in type 1 collagen. Bone and teeth are affected, but other type 1 collagen-rich tissues, such as skin, are not affected. Osteogenesis imperfecta has been reported in dogs, cats, cattle, sheep, mice and tigers. Defects in the COL1A1 and COL1A2 genes have been found in the Golden retriever dog and mice.
JPC Diagnosis:
1. Bone, distal radius and ulna: Osteopenia, epiphyseal, focally extensive, marked, with metaphyseal reactive woven bone formation (fracture callus), synoviocyte hyperplasia, and pannus.
2. Bone, proximal humerus: Osteosclerosis, epiphyseal and metaphyseal, focally extensive, marked, with growth retardation lattices, mild trabecular woven bone formation, mild marrow fibrosis and myxomatous metaplasia, and focally extensive periosteal granulation tissue.
Conference Comment:
Additionally, many conference participants received a section of bone interpreted as proximal humerus instead of the distal radius and ulna described by the contributor. In contrast to the epiphyseal osteopenia noted in the radius, the primary lesion in the proximal humerus is osteosclerosis attributed to the persistence of primary spongiosa with increased cross connections between cartilage spicules resulting from decreased osteoclasis (growth retardation lattices). Throughout the metaphysis, there are scattered trabeculae of woven bone, and within the metaphyseal bone marrow there is mild fibrosis and/or myxomatous metaplasia. In the adjacent periosteum, there is focally extensive granulation tissue.
Osteogenesis imperfecta, though well-described in humans, remains a rare diagnosis in animals. As mentioned by the contributor, most cases of OI stem from mutations in COL1A1 and/or COL1A2 genes that encode the α1 and α2 chains of type I collagen, respectively. Collagen in bone, dentin, ligaments, tendons, and the sclera is primarily type I, resulting in the characteristic confinement of lesions to these anatomic locations.(1-6) While type I collagen also predominates in the skin, OI usually does not produce cutaneous lesions,(6) although skin fragility has been reported in affected newborn New Zealand Romney lambs.(1)
Noteworthy, OI represents a heterogenous group of diseases, and defects in glycosaminoglycan and proteoglycan metabolism(2) or deficient osteonectin synthesis(6) may also account for some cases. This observation, combined with evidence that a wide variety of mutations in COL1A1 and/or COL1A2 have been demonstrated in humans with OI, explains the substantial phenotypic variation seen in the disease.(1) The conference moderator emphasized this point and noted that because mutations that produce OI can cause qualitative and/or quantitative collagen defects, the bone fragility that results is not necessarily related to bone mass, and thus a uniform set of pathognomonic microscopic findings cannot be relied upon for the diagnosis of OI. As a result, the definitive diagnosis of OI based solely on the tissues provided for histologic examination in this case is not possible, although the clinical history in combination with the gross and microscopic findings is certainly suggestive of the disease. As described by the contributor, the growth plates in this case are microscopically normal, an expected finding in OI since the collagen found in cartilage is primarily type II.(6)
The osteosclerotic lesions in the proximal humeral metaphysis and epiphysis led some participants to consider osteopetrosis in the differential diagnosis, and the conference moderator noted that osteopetrosis, like OI, may manifest as brittle bones prone to fracture. Osteopetrosis in cats has been associated with feline leukemia virus (FeLV) infection and chronic vitamin D toxicosis.(5) However, osteopetrosis classically results in a more severe failure of bone modeling than is present in this case. Additionally, although trabecular bone in OI is often described as microscopically normal or osteopenic, some cases, particularly those seen in lethal OI with skin fragility in New Zealand Romney lambs, result in persistent trabeculae of calcified cartilage that extend into long bone diaphyses and fill marrow cavities, accompanied by a paucity of osteoclasts, similar to osteopetrosis.(1,5)
Of note, some of the earliest cases of OI in dogs and cats were later shown to represent misdiagnoses of fibrous osteodystrophy (FOD) due to nutritional secondary hyperparathyroidism, with apparent familial susceptibility actually derived from exposure to common nutritional deficiencies.(2) The paucity of osteoclasts in this case argues against a diagnosis of FOD. Other considerations briefly discussed during conference included osteopenia secondary to protein calorie malnutrition and lack of osteoclasts due to inconsistent exposure to such antiosteoclastic agents as lead or bisphosphonates. In addition, the lesion in the distal metaphyses of the radius and ulna is somewhat reminiscent of the scorbutic lattice of vitamin C deficiency (scurvy). However, conference participants uniformly agreed that there is more osteoid deposition on the primary spongiosa than would be expected in scurvy. Furthermore, the growth retardation lattice noted in the proximal humeral metaphysis is not expected in scurvy, and this case lacks other classic findings of scurvy, such as microfractures or infractions with associated hemorrhage and fibrin.(5,6)
References:
2. Denholm LJ, Cole WG: Heritable bone fragility, joint laxity and dysplastic dentin in Fresian calves: a bovine syndrome of osteogenesis imperfecta. Aust Vet J 60:9-17, 1983
3. Leeb F, Peters M, Brugmann M, Fehr M, Hewicker-Trautwein M: Osteogenesis imperfecta in two litters of dachshunds. Vet Pathol 40:530-539, 2003
4.Seeliger F, Leeb T, Peters M, Brugmann M, Fehr M, Hewicker-Trautwein M: Osteogenesis imperfecta in two litters of Dachshunds. Vet Pathol 40:530-539, 2003
5. Thompson K: Bones and joints. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 33-38. Saunders Elsevier, Philadelphia, PA, 2007
6.Weisbrode SE: Bones and joints. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 1065-1066. Mosby Inc., St. Louis, MO, 2007