Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 13
2 January, 2018
CASE II: N1301/17B: (JPC 4105937).
Signalment: 4.5 years, male-neutered, Domestic Shorthaired, Felis catus, cat
History: A 4.5 year-old male-neutered cat was submitted to post mortem examination. Found dead unexpectedly.
Gross Pathology: The cat weighed 4.5 kg and was in a good body condition with adequate subcutaneous and intraabdominal fat deposits. Skeletal musculature of both hind limbs was mottled pale to red. The thoracic cavity contained 30 ml of translucent serosanguinous fluid. The trachea contained moderate amounts of white froth and both lungs were diffusely heavy and wet with copious frothy fluid on cut surface. The heart weighed 35g and the left atrium was subjectively enlarged. The left to right free ventricular wall ratio was 3:1 (9:3 mm). The septum measured 9 mm in thickness. Located at the aortic bifurcation was a 3 cm plug of friable dark red to tan material, completely occluding the vessel lumen and extending along both iliac arteries. Thyroid glands were bilaterally unremarkable.
Laboratory results: None.
Microscopic Description:
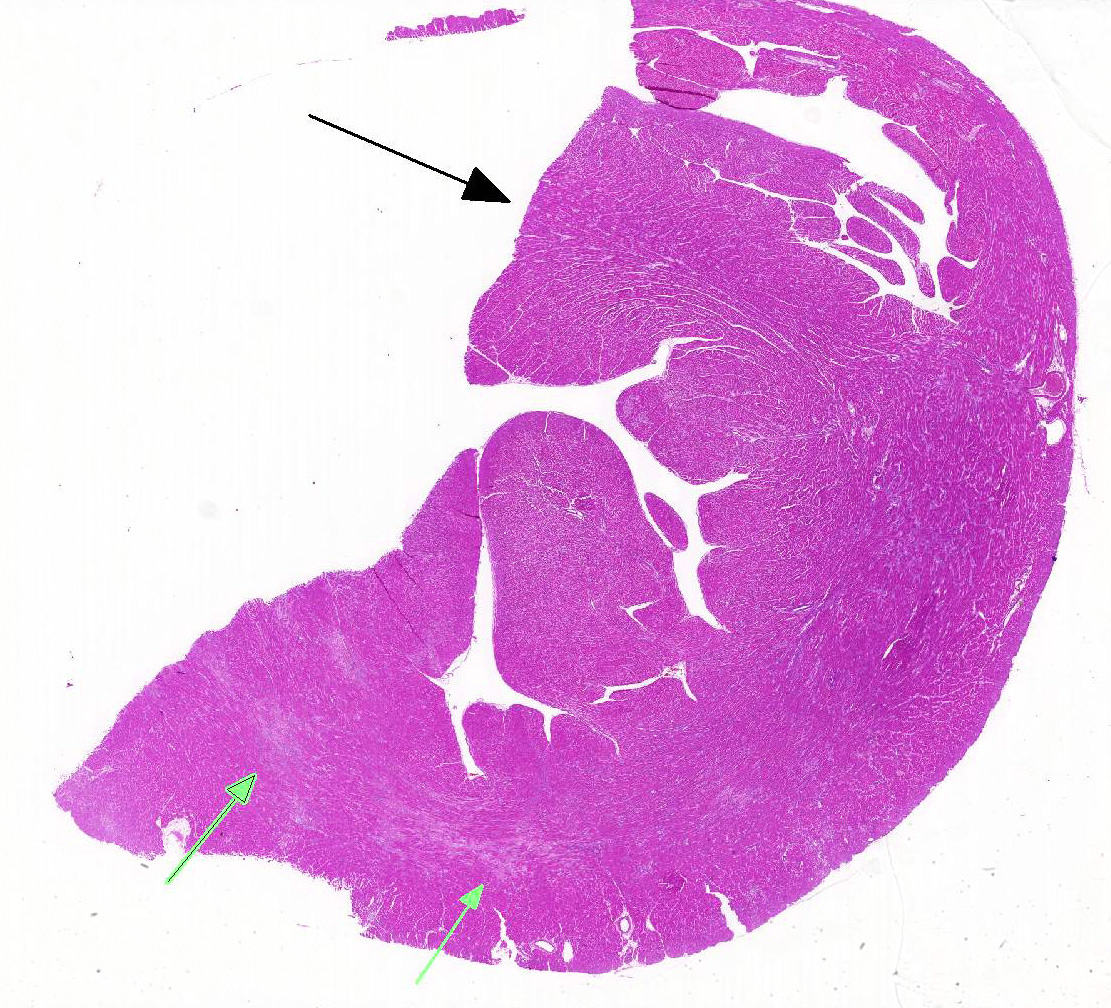
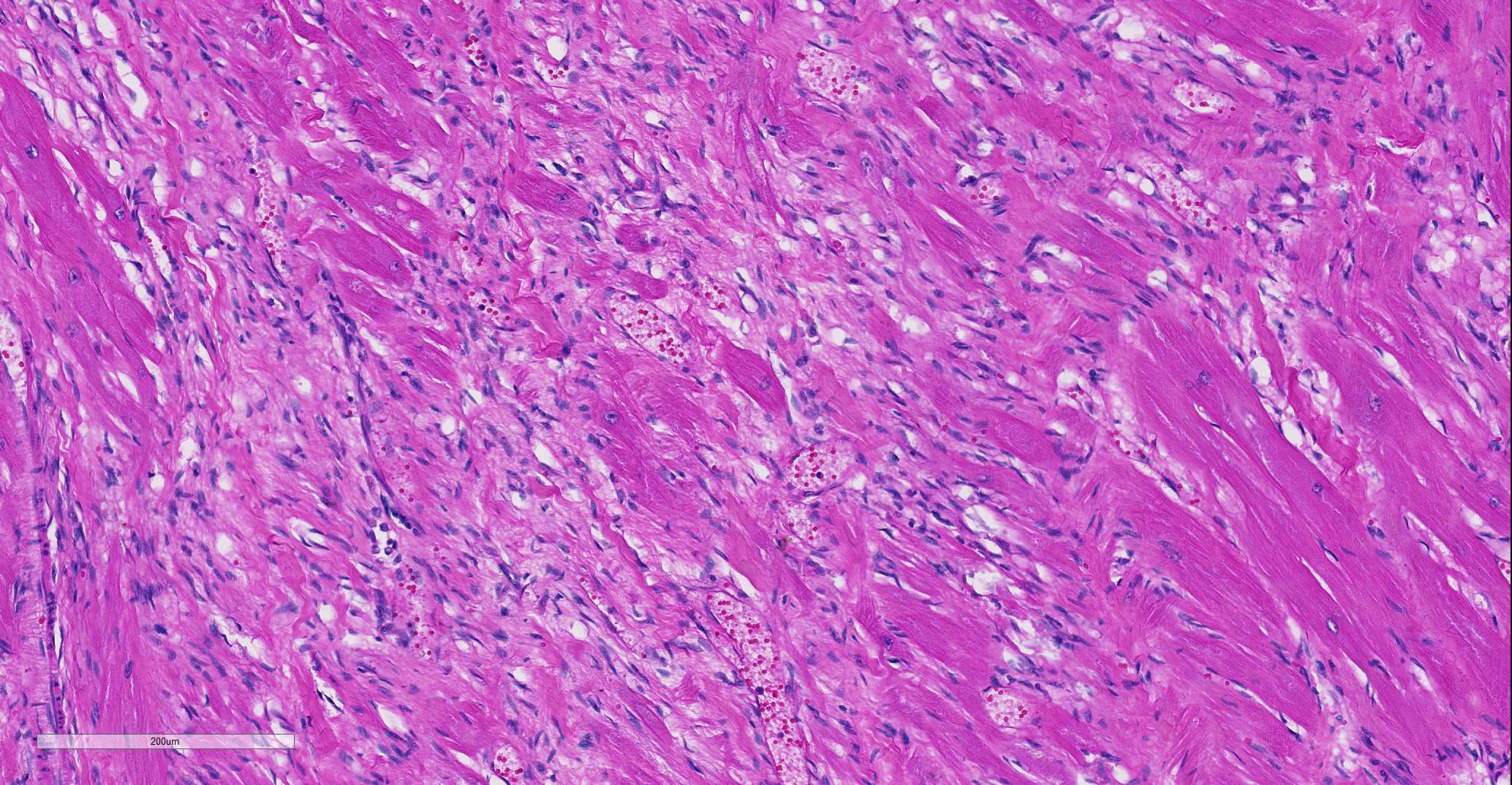
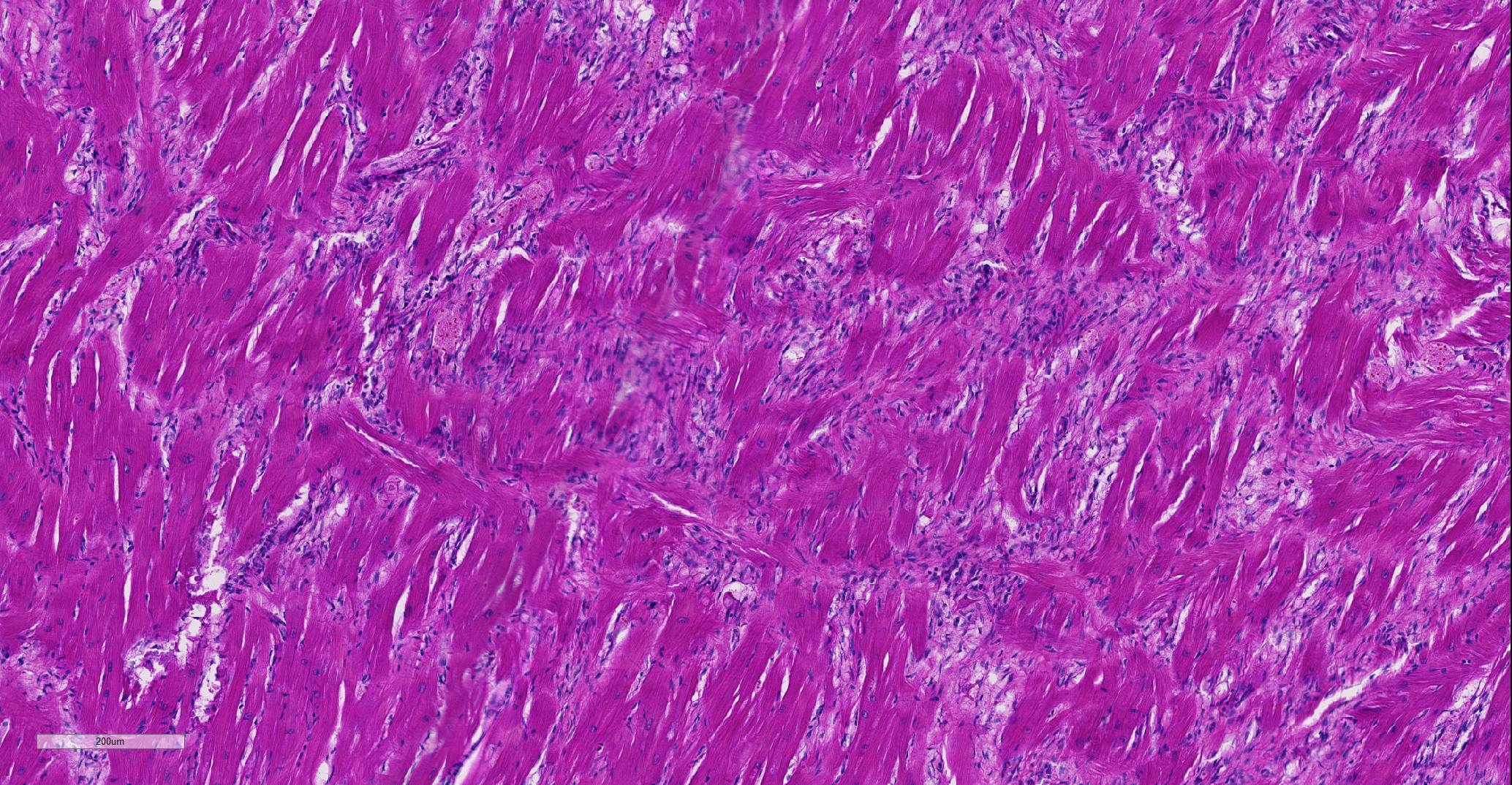
Heart: Affecting approximately 10-20% of the myocardium of the left ventricular free wall are broad bands of mature and developing fibrous tissue extensively replacing and dissecting between cardiac myocytes. Myocytes entrapped within fibrous tissue are often shrunken and hypereosinophilic and have lost cross-striation (segmental degeneration). Fibrosis affects mainly the middle aspect of the left free ventricular wall and smaller foci within the septum. Furthermore, cardiac myocytes of the left ventricle are multifocally arranged in an interwoven pattern (myofiber disarray). Within the left ventricular wall and extending into the right ventricular wall, myocytes frequently measure 2-3 times normal diameter and show vesicular nuclei (hypertrophy).
Contributor’s Morphologic Diagnoses:
Heart: Hypertrophic cardiomyopathy, domestic shorthaired cat, Felis catus.
Contributor’s Comment: Hypertrophic cardiomyopathy (HCM) is the most frequent feline cardiomyopathy, accounting for almost two thirds of cases.3 In affected cats, a thickened and remodeled ventricular cardiac muscle, unable to relax completely during diastole, leads to incomplete ventricular filling and thus a decreased preload (diastolic dysfunction).2 Common clinical signs include lethargy, heart murmurs, tachycardia and/or may be secondary to pulmonary oedema (dyspnea, coughing) or thromboembolism (hindlimb paresis).1,3,9 Sudden death may also occur.1
This case illustrates classical features of idiopathic feline HCM, including subsequent aortic thromboembolism. At 35g, the heart was markedly heavier than normal in adult cats (<20-25g) and within the upper range of values reported for cats that died of HCM (29-37g).2 As in the present case, symmetrical and concentric hypertrophy of the myocardium is most common, thus retaining a ‘normal’ left:right ventricular free wall ratio of 3:1, while wall thicknesses are absolutely increased.
Alterations in blood flow and velocity, especially within the often dilated left atrium, may lead to turbulence and produce focal thrombi, which may subsequently be flushed into circulation, where they become lodged most frequently at the aortic-iliac junction.9 At this location, thromboembolism leads to acute hindlimb paraparesis with clinical signs classically termed the five ‘p’s (paresis-pain-pallor-pulselessness-poikilothermy), which may be the first presenting clinical sign of a cat with HCM.10 Renal cortical infarcts may also be a feature indicating previous thromboembolism of renal arteries (not present in this case).
Histological hallmarks of feline HCM are hypertrophied cardiac myocytes with vesicular nuclei, myofiber disarray, especially within the left free ventricular wall and septum, and replacement fibrosis, all of which can be observed in this case.9 Further, medial hypertrophy of intramyocardial arteries with or without perivascular mononuclear infiltrates may be present.9 If performed antemortem, immunoassays for cardiac biomarkers often reveal increased levels of cardiac troponin I, indicating ongoing myocardial damage.4 A familial predisposition for HCM is best characterized in the Maine Coon and Ragdoll breeds.7,8 Two separate mutations have been identified affecting the gene coding for myosin binding protein C3 (MYBPC3): an alanine for proline substitution in exon 3 of the MYBPC3 gene was identified as the causative mutation in Main Coons, whereas in Ragdolls, an arginine to tryptophan mutation in codon 820 occurs, indicating that mutation events occurred independently from each other in each breed.7,8 While hypertrophy of the cardiac muscle also occurs secondarily in cases of hyperthyroidism, this entity should not be confused with primary (idiopathic) HCM.5 More recently, feline panleukopenia virus has been implicated as a possible cause of HCM in cats.6
Contributing Institution:
UCD School of Veterinary Medicine, University College Dublin, Belfield, Dublin 4, Ireland
http://www.ucd.ie/vetmed/
JPC Diagnosis: Heart, myocardium: Fibrosis, multifocal, moderate, with myofiber hypertrophy, disarray, degeneration and loss.
JPC Comment: Hypertrophic cardiomyopathy (HCM), first diagnosed in humans in the 1950’s is an autosomal dominant primary myocardial disease with incomplete penetrance6 that affects approximately 1 in every 500 humans. It is characterized by increased left ventricular mass in the absence of a metabolic cause or pressure overload. Causative mutations for this disease have been identified as single base substitutions in a variety of sarcomeric genes, including beta-myosin heavy chains, cardiac myosin binding protein C, cardiac troponins T, I and C, alpha-tropomyosin, the essential and regulatory light chains, actin, and titin.7 In humans, the two most common genes affected by HCM mutations are the myosin heavy chain gene MYH7 (the gene that encodes for the motor protein beta-myosin heavy chain - - the sarcomeric protein that splits ATP to generate force) and the cardiac myosin binding protein C (cMyBP-C) – the same defective protein as seen in Maine Coon and ragdoll cats with HCM.6 In the human, approximately 70% of cases are the result of 11 identified sarcomeric genes, suggesting other mutations in proteins are associated with sarcomeric function. A similar case exists in Maine Coon cats, as not all affected individuals demonstrate known mutations.1
Cardiac troponin I (cTnI) is commonly used as a measurement for ongoing myocardial damage in cats (and humans) with HCM; this protein, which inhibits the structural interaction of the myosin heads with the actin-binding sites in cardiac and skeletal muscle, has been shown to increase rapidly in the serum after cardiomyocyte injury and is a sensitive and specific marker for cats with moderate to severe HCM as compared to normal cats whether or not congestive heart failure is present. Although there is still some debate, increased levels of cTnI are generally consistent with irreversible myocardial damage. 5
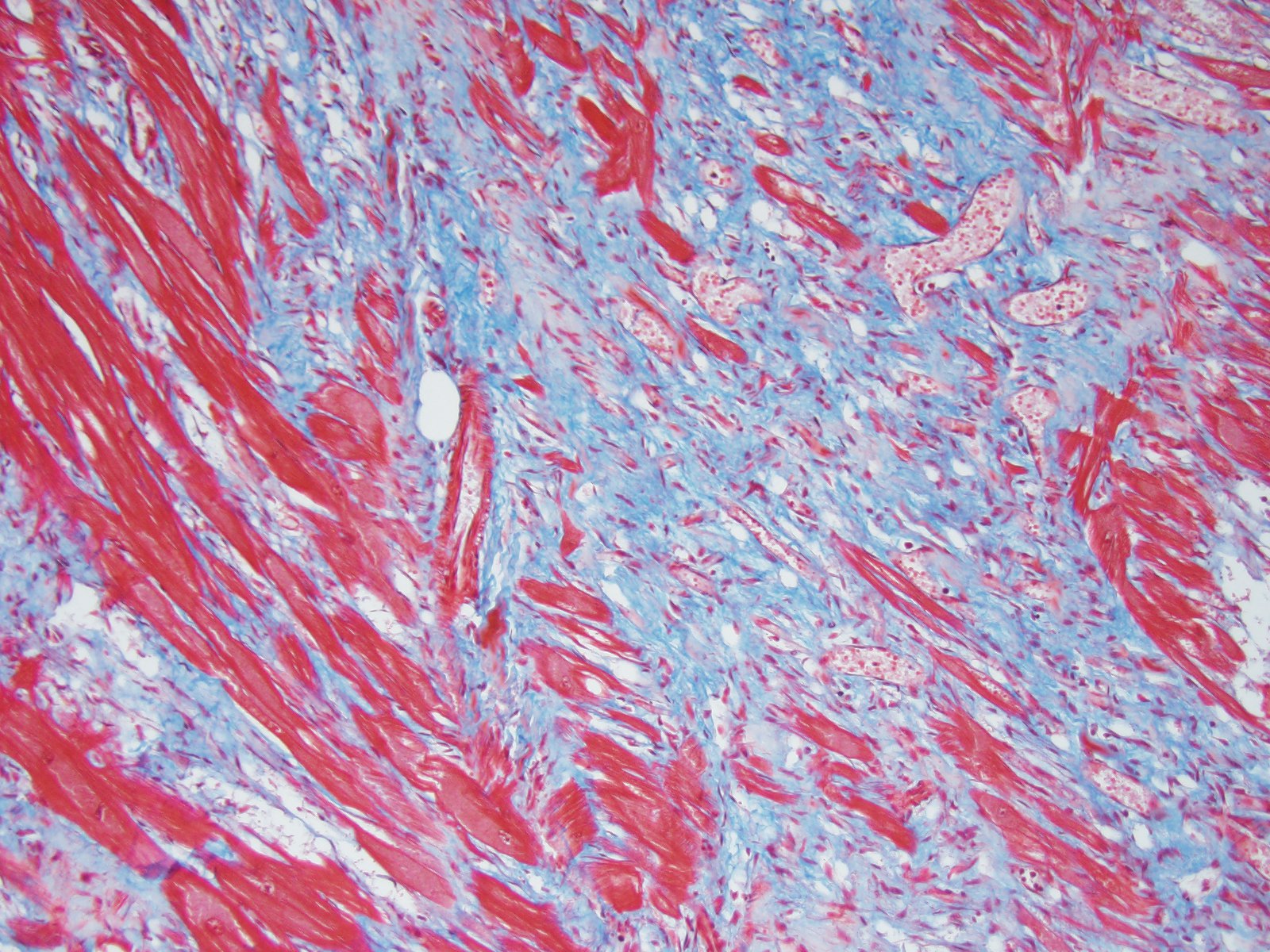
One of the characteristic histologic changes associated with HCM is myocardiocyte loss and fibrosis, which often starts in proximity to myocardial vessels. A number of vascular changes have been identified in cats and humans with HCM, to include microvascular or intramural coronary arterial disease, which likely result in areas of ischemic damage and resulting fibrosis, initially localized to perivascular areas within the myocardium.5 In humans with HCM, abnormal coronary flow dynamics, decreased coronary artery diastolic reserve, and systolic compression of the septal perforator arteries have been documented as contributory to myocardial ischemia. Furthermore, the increase in myocardial muscle mass without compensatory increase in myocardial capillary density is also likely to contribute to ischemic damage in the myocardium of affected individuals.4
Since 1960, surgical intervention and septal myomectomy have been common approaches to relieving left ventricular outflow tract obstruction. Alcohol septal ablation (a less invasive procedure in which ethanol is injected to the first or second perforator artery, resulting in localized myocardial infarction) was introduced in 1994, and its surgical complication rate has proven to be similar to that of surgical myomectomy. 4
During the conference, the moderator cautioned about interpretation of luminal diameter in these cases, based on a lack of knowing the level of section within the heart as well as the status of rigor during fixation.
In the normal heart, myofibers are often maloriented to each other at the area in which ventricles meet the interventricular septum – this may be misinterpreted. The described feature of ‘myofiber disarray” in HCM is most appropriately evaluated wholly within the left ventricular free wall or septum. In cases of microscopic examination of the heart, it would be unusual to be able to take a cross=section through the ventricles and septum and not find disarrayed fiber, but emphasized the need to take multiple sections to completely evaluated the heart. Without the presence of the disarrayed fibers, a diagnosis of HCM cannot be made; the hypertrophic changes seen in a number of fibers such as markedly enlarged nuclei are non-specific and may be seen in a number of heart diseases.
Microscopically, the moderator briefly discussed the differentiation between “interstitial” and “replacement” fibrosis for complete description of this lesion, and highlighted the need for a Masson’s trichrome to fully appreciate the amount of fibrosis in this case.
References:
- Abbott JA. Feline hypertrophic cardiomyopathy: an update. Vet Clin North Am Small Anim Pract 2010; 40(4):685-700.
- Côté E, MacDonald KA, Meurs KM, Sleeper MM. Feline Cardiology. 1st ed. Wiley-Blackwell, West Sussex, UK, 2011:110-112.
- Ferasin L, Sturgess CP, Cannon MJ, Caney SM, Gruffydd-Jones TJ, Wotton PR. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994-2001). J Feline Med Surg 2003; 5(3):151-159.
- Hang DH, Nguyen A, Schaff HY. Surgical treatment of hypertrophic cardiomyopathy: a historical perspective. Ann Cardiothor Surg 2017; 6(4):318-328.
- Herndon WE, Kittleson MD, Sanderson K, Drobatz KJ, Clifford CA, Gelzer A, Summerfield NJ, Linde A, Sleeper MM. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002; 16(5):558-564.
- Kittleson MD, Meurs K, Harris S. The genetic basis of hypertrophic cardiomyopathy in cats and humans. J Vet Cardiol 2015; 17(Suppl 1):S53-S73.
- Liu SK, Peterson ME, Fox PR: Hypertropic cardiomyopathy and hyperthyroidism in the cat. J Am Vet Med Assoc 1984; 185(1):52-57.
- Meurs KM, Fox PR, Magnon AL, Liu S, Towbin JA. Molecular screening by polymerase chain reaction detects panleukopenia virus DNA in formalin-fixed hearts from cats with idiopathic cardiomyopathy and myocarditis. Cardiovasc Pathol 2000; 9(2):119-126.
- Meurs KM, Norgard MM, Ederer MM, Hendrix KP, Kittleson MD. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 2007; 90(2):261-264.
- Meurs KM, Sanchez X, David RM, Bowles NE, Towbin JA, Reiser PJ, Kittleson JA, Munro MJ, Dryburgh K, Macdonald KA, Kittleson MD. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet; 2005 14(23):3587-3593.
- Robinson WF, Robinson NA. Cardiovascular System. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. 6th ed. Elsevier, St. Louis, MO, USA, 2016:46-47.
- Smith SA, Tobias AH. Feline arterial thromboembolism: an update. Vet Clin North Am Small Anim Pract; 2004; 34(5):1245-1271.