Wednesday Slide Conference, 2025-2026, Conference 1, Case 4
Signalment:
5-year-old, intact male, Criollo horse Equus caballus.History:
The horse had a 3-day history of progressive neurological signs including lethargy, circling, recumbency, paralysis, and occasional seizures, and was humanely euthanized due to severe clinical deterioration and poor prognosis.Gross Pathology:
Gross findings included flattening of gyri in the cerebral hemispheres, diffuse hyperemia of the cerebral leptomeninges, and scattered foci of hemorrhage in the gray matter of the spinal cord.Microscopic Description:
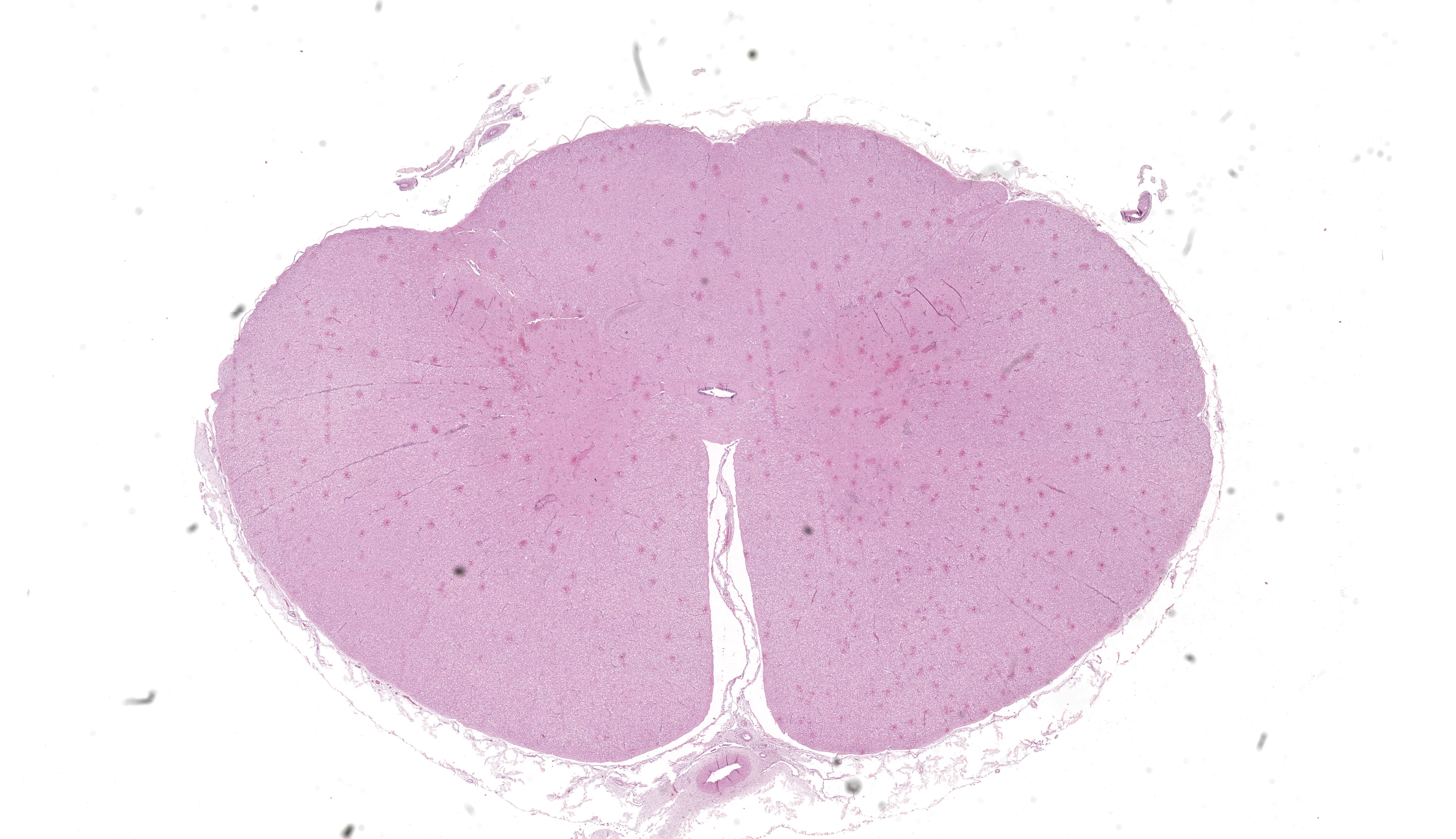
Samples of brain and spinal cord were positive for Western equine encephalitis virus (WEEV) by RT-PCR. Spinal cord (C1): multifocally the perivascular spaces are expanded and infiltrated by lymphocytes, histiocytes and fewer plasma cells forming layers of up to 7 cell thick (perivascular cuffing), with occasional accumulation of perivascular eosinophilic homogeneous finely granular material (protein-rich edema fluid. Similar inflammatory cells multifocally infiltrate the adjacent neuropil and/or white matter, occasionally forming discrete nodular aggregates. Rarely, multinucleated cells with abundant eosinophilic cytoplasm (interpreted as multinucleated astrocytes) are admixed with the inflammatory infiltrates. There is mild to moderate erythrocyte extravasation into the neuroparenchyma (hemorrhage). The inflammation, edema and hemorrhages are more frequent and severe in the gray than the white matter, with hemorrhages being particularly prominent in the dorsal horns. Scattered neurons depict chromatolysis (neuronal necrosis) and are either surrounded by inflammatory/glial cells (satellitosis) or invaded by them (neuronophagia) (not present in all sections). Capillaries are frequently hyperemic and lined by plump endothelial cells. Few swollen axons within dilated myelin sheaths (spheroids) are observed in the white matter. The leptomeninges are multifocally infiltrated by lymphocytes, histiocytes and fewer plasma cells.Contributor's Morphologic Diagnoses:
Spinal cord (C1): Meningomyelitis, lymphocytic, histiocytic and plasmacytic, multifocal, moderate, with neuronal necrosis and hemorrhage, horse.Contributor's Comment:
Differential diagnoses for non-suppurative encephalitis/encephalomyelitis in horses include viruses [neuropathogenic strains of Equine herpesvirus 1 (EHV 1), rabies virus, several viruses in the Flavivirus genus such as West Nile virus (WNV), Japanese encephalitis virus (JEV), Saint Luis encephalitis virus (SLEV), and alphaviruses (Eastern, Western and Venezuelan equine encephalitis viruses: EEEV, WEEV and VEEV, respectively)], as well as protozoal diseases (Sarcocystis neurona, Neospora hughesi)4,9,10,15.Given that most viral causes of encephalitis in horses are zoonotic and/or notifiable to the World Organization for Animal Health, rapid confirmation of the etiologic diagnosis is crucial. This can be achieved by PCR, immunohistochemistry, or in situ hybridization targeting specifically the different viruses, by virus isolation, next generation sequencing, and/or by fluorescent antibody testing in the case of rabies.
While JEV is exotic in South America, neuropathic EHV 1, WNV, SLEV, WEEV, EEEV, and rabies are endemic or sporadically reported1,5,6,11-13. In the spring of 2023 and summer of 2024, a large epizootic outbreak of WEE affecting primarily horses and to a lesser extent humans occurred in South America, notably in Argentina,16 Uruguay,17 and southern Brazil3. The case presented here was the first equine case investigated in Uruguay during this epizootic in the summer of 2024. The epidemiological, clinical, and histopathologic findings lead to the suspicion of a viral encephalitis, and WEE was highly suspected as cases had recently been confirmed in neighboring areas of Argentina. WEEV was detected by reverse transcriptase PCR in samples of spinal cord and brain from this horse submitted to the laboratory of the national sanitary authority (?Ministerio de Ganadería, Agricultura y Pesca? (MGAP), confirming the etiologic diagnosis.
Besides WEEV, VEEV and EEEV are other important alphaviruses (Togaviridae family), WEEV being the less virulent for horses of the three. Reservoir hosts are mainly birds (WEEV, EEEV), and rodents (VEEV)13. WEEV is transmitted primarily by Culex and Aedes mosquitoes from reservoir hosts to horses and humans, which are considered dead-end hosts.
The pathogenesis of alphavirus infection and encephalitis is complex and involves viremia after replication in peripheral tissues, neuroinvasion, and viral spread within the central nervous system. The initial viremia often goes unnoticed (subclinical), or manifests as fever and depression. If the animal fails to recover, neuroinvasion occurs, after which the virus replicates in neurons, glial cells, and blood vessels of the central nervous system. At this stage, the animal usually exhibits neurological clinical signs such as circling, central blindness, seizures, and terminal paralysis14. There are usually no gross changes, while histologic lesions frequently localize in the cerebral cortex and are almost limited to the gray matter, typically with prominent neutrophil infiltration when the course is short or lymphoplasmacytic infiltration after a couple of days. Other common findings are neuronal degeneration, neuronophagia, gliosis, edema, and hemorrhage4. The histologic lesions caused by these three alphaviruses are similar in nature and distributions. For this reason, specific testing (i.e. PCR, IHC, ISH, and/or virus isolation) is required to confirm the etiologic diagnosis.
Outbreaks of arboviral-encephalitis usually affect non-vaccinated horses10. As vaccination is not mandatory in South America, many horses are not routinely vaccinated against these diseases.
Contributing Institution:
Instituto Nacional de Investigacion Agropecuaria (INIA), Uruguay. Avenida Italia 6201, Edificio Los Guayabos, Parque Technologico del LATU, Montevideo, Uruguay www.inia.uyJPC Diagnoses:
Cervical spinal cord: Poliomyelitis, lymphohistiocytic, diffuse, mild, with multifocal neuronal degeneration.JPC Comment:
Last year's WSC coordinator, MAJ James Gaffney, adorned in a black cowboy hat provided by COL Daye for case presenters, grabbed this spinal cord case by the dorsal horns and proclaimed, Pardner, this slide ain't big enough for the two of us; In the end, the slide was wrassled and conference participants branded this a "poliomyelitis" due to the focus of the inflammation being within the grey matter of the spinal cord. This case stimulated great discussion about the need for pathologists and clinicians alike to pay special attention to equine neurologic cases for which viral differentials are considered, as every virus on the differentials list is reportable and almost all are zoonotic. This list included flaviviruses (West Nile virus, Japanese encephalitis virus, Saint Louis encephalitis virus), the equine alphaviral encephalidities (WEEV, EEEV, and VEEV), equine herpesvirus-1, and rabies virus. In cases where any of these viral etiologies is suspected, rapid reporting and confirmatory diagnosis are crucial.
WEEV is widely considered to have arisen from a recombination event between Eastern equine encephalitis virus (EEEV) and a Sindbis-like alphavirus that resulted in a viral chimera. This recombinant virus ultimately diversified into WEEV, Highlands J Virus (HJV) and Fort Morgan virus (FMV), all of which are considered part of the WEEV antigenic complex. All three of these WEEV strains are considered endemic to North America2.
The first major outbreak of WEEV in the United States occurred in the 1940s with approximately 500,000 equine cases and several thousand human infections. The disease resurged with an additional outbreak in the United States and Canada the 1980s. Since then, cases in the United States have dropped significantly. However, Uruguay and Argentina experienced another outbreak of WEEV in 2024, with both equines and humans affected, bringing WEEV back to the forefront of disease surveillance. WEEV causes disease through a variety of factors. Its structural proteins play significant roles in its survivability and pathogenesis and include a capsid protein, a signal peptide (E3), an envelope glycoprotein (E2) that binds target receptors, another envelope glycoprotein (E1) that primarily functions to fuse the virus with the cellular membrane of the target cell, and a signaling peptide (6K) that may also function as a viroporin6. These structural proteins continue to be targets in WEEV vaccine research.
Target cell receptors of WEEV have historically included protocadherin 10 (PCDH10), Very Low-Density Lipoprotein Receptor (VLDLR), and apolipoprotein E receptor 2 (ApoER2), all of which are expressed by neurons in the CNS7. However, the binding affinity for these receptors has changed significantly over time and has played a role in the submergence of WEEV in North America. A newer strain of WEEV has, for example, lost the ability to bind mammalian PCDH10, but continues to bind avian PCDH107. This changing affinity for receptors across species keeps those that study WEEV on their toes and provides a sentinel for potential re-emergence of WEEV. Conference discussion touched on some of these evolutions as part of the viral ecology that led to the 2024 resurgence of WEEV in South America. Rapid genetic changes, in concert with increased rainfall leading to booming mosquito populations, bird migrations providing ample reservoirs, lower vaccination rates against WEEV in horses, and surveillance gaps due to the disease being considered historic in South America all likely contributed to the outbreak and are a prime example of how rapidly deadly viruses can re-emerge in the face of ideal ecologic conditions.
References:
- Albrieu-Llinas G, Gallardo R, Konigheim BS, et al. Arbovirus serosurvey (Orthobunyavirus, Flavivirus, and Alphavirus) in a draft horse population from Santa Fe, Argentina (2013-2016). Arch Virol. 2021;166(3):881–884.
- Allison AB, Stallknecht DE, Holmes EC. Evolutionary genetics and vector adaptation of recombinant viruses of the western equine encephalitis antigenic complex provides new insights into alphavirus diversity and host switching. Virology. 2015;474:154-62.
- Campos AS, Franco AC, Godinho F, et al. Molecular epidemiology of Western equine encephalitis virus in Brazil, 2023-2024. Version 1. medRxiv. Preprint 2024 Apr 18.
- Cantile C, Youssef S. Nervous system. In: Maxie M.G., ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 1. 6th ed. St. Elsevier; 2016.
- Delfraro A, Burgueno A, Morel N, et al. Fatal human case of Western equine encephalitis, Uruguay. Emerg Infect Dis. 2011;17(5):952–954.
- Gauci PJ, Wu JQ, Rayner GA, Barabé ND, Nagata LP, Proll DF. Identification of Western equine encephalitis virus structural proteins that confer protection after DNA vaccination. Clin Vaccine Immunol. 2010;17(1):176-9.
- Li W, Plante JA, Lin C. et al. Shifts in receptors during submergence of an encephalitic arbovirus. Nature. 2024;632: 614–621.
- Mori E, Borges AS, Delfiol DJZ, et al. First detection of the equine herpesvirus 1 neuropathogenic variant in Brazil. Rev Sci Tech Off Int Epiz. 2011;30(3):949–954.
- Rech R, Barros C. Neurologic diseases in horses. Vet Clin North Am Equine Pract. 2015;31(2):281–306.
- Rosa R, Costa EA, Marques RE, et al. Isolation of Saint Louis encephalitis virus from a horse with neurological disease in Brazil. PLoS Negl Trop Dis. 2013;7(11):e2537.
- Silva ASG, Matos ACD, da Cunha MACR, et al. West Nile virus associated with equid encephalitis in Brazil. Transbound Emerg Dis. 2019;66(1):445–453.
- Silva ML, Galiza GJ, Dantas AF, Oliveira RN, Iamamoto K, Achkar SM, Riet-Correa F. Outbreaks of Eastern equine encephalitis in northeastern Brazil. J Vet Diagn Invest. 2011;23(3):570–575.
- Silva Oliveira FA, Souza Castro RJ, de Oliveira JF, et al. Geographical and temporal spread of equine rabies in Brazil. Acta Trop. 2022;227:106302.
- Steele KE, Twenhafel NA. Review Paper: Pathology of animal models of alphavirus encephalitis. Vet Pathol. 2010;47:790.
- Wobeser BK, Godson DL, Rejmanek D, Dowling P. Equine protozoal myeloencephalitis caused by Neospora hughesi in an adult horse in Saskatchewan. Can Vet J. 2009;50(8):851–853.
- World Health Organization. Disease outbreak news. Western equine encephalitis - Argentina. December 28, 2023. Accessed June 5, 2024.
- World Health Organization. Disease outbreaks news. Western equine encephalitis - Uruguay. February 8, 2024. Accessed June 5, 2024.