Signalment:
Adult male cynomolgus macaque (
Macaca fascicularis).This macaque was
in a study to determine the efficacy of a novel therapeutic drug for treating
Marburg virus (MARV) infection. All of the monkeys in this study were
inoculated subcutaneously with MARV and then once daily intramuscular
treatments with either saline (control group) or different doses of the
therapeutic drug (experimental groups) began. This animal was in one of the
experimental groups and it was found dead on Day 11 after viral challenge.
This monkey was
part of a research project conducted under an IACUC approved protocol in
compliance with the Animal Welfare Act, PHS Policy, and other federal statutes
and regulations relating to animals and experiments involving animals. The
facility where this research was conducted is accredited by the Association for
Assessment and Accreditation of Laboratory Animal Care, International and
adheres to principles stated in the 8
th edition of the Guide
for the Care and Use of Laboratory Animals, National Research Council, 2011.
Gross Description:
The mucosa of
the rectum and distal 20 cm of the colon was diffusely hemorrhagic. The liver
was enlarged (~1.5 X), pale tan, and markedly friable. The spleen was also
friable. Other organs were unremarkable.
Histopathologic Description:
Lung
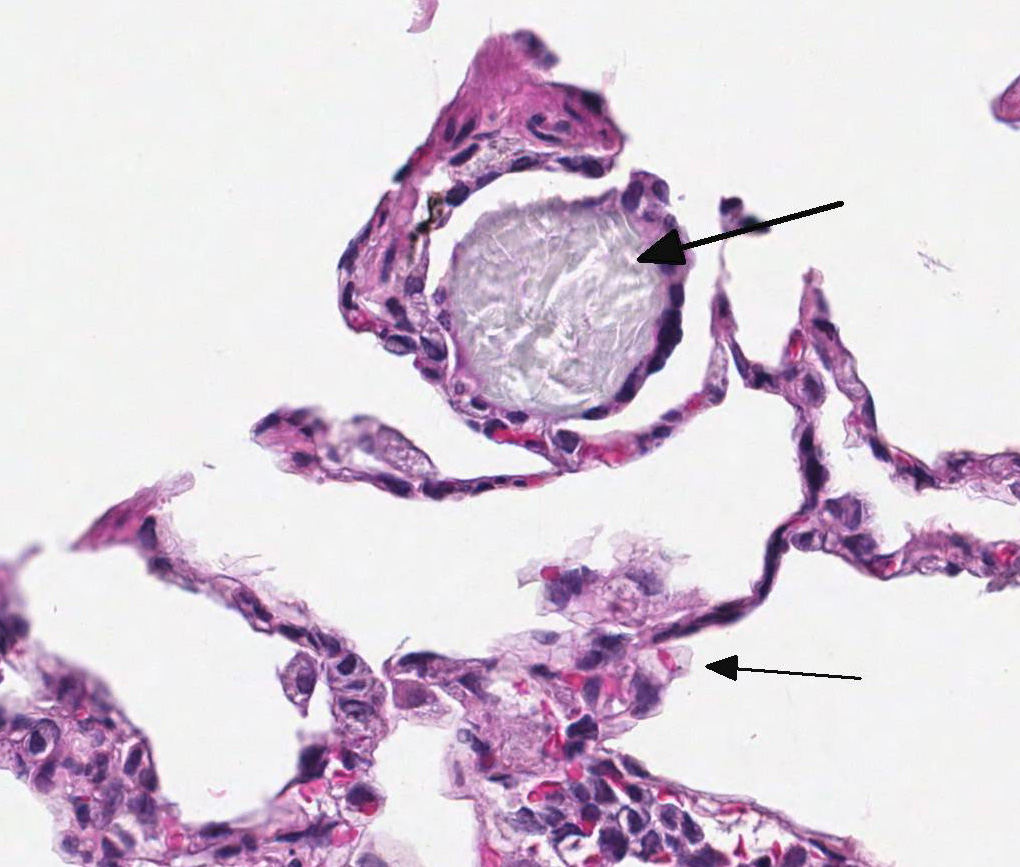
(right inferior lobe): Multifocally within alveoli and often attached to the
alveolar septa, there are low numbers of multinucleated giant cells, measuring
up to 100 µm in diameter, most of which contain intra-cytoplasmic aggregates of
pale blue-gray amorphous to spicular refractile material. The interstitium
also contains scattered aggregates of low numbers of histiocytes containing
intracytoplasmic brown-black finely-granular material. Many blood vessels
contain numerous intraluminal mononuclear leukocytes (monocytes).
Morphologic Diagnosis:
1. Lung;
intravascular leukocytosis (monocytic), moderate
2. Lung; multifocal
histiocytic (multinucleated giant cell) alveolitis, mild, with intracytoplasmic
crystalline foreign bodies
3. Lung; multifocal
interstitial anthracosilicosis, minimal
Lab Results:
None
Condition:
Histiocytic alveolitis with intracytoplasmic crystalline protein
Contributor Comment:
The
timing of this monkeys death is within the usual interval (i.e. 7-11 days)
that cynomolgus macaques die after experimental exposure to a lethal dose of
MARV. There were histologic lesions in the liver, spleen, adrenal glands,
tonsils, and lymph nodes of this monkey that were caused by MARV infection;
these organs are considered target organs for the virus.
7 Immuno-histochemistry
(IHC) revealed MARV antigen in every organ examined from this animal. The
histologic findings and IHC results confirmed that this macaque died from a
disseminated MARV infection. Although the exact cause of the intestinal bleeding
noted at necropsy was not determined, this was most likely associated with
MARV-induced coagulopathy; disseminated intravascular coagulopathy (DIC) occurs
commonly in primates (including humans) infected with viruses in the
family
Filoviridae (i.e. ebolaviruses and MARV).
6,7
The monocytic
leukocytosis noted within pulmonary blood vessels of this monkey is
attributable to the viral infection. IHC revealed abundant MARV antigen in
many of these monocytes. Cells of the monocyte-macrophage system are infected
very early during the course of filovirus infection and are primarily
responsible for disseminating the viruses throughout the body.
5,7
The presence of
multinucleated giant cells within alveoli and/or attached to alveolar septa of
this monkey was an unexpected finding and was unrelated to the MARV infection.
This lesion was seen in the right inferior lung lobe but not in the other lung
lobes that were examined histologically. These giant cells were an inflammatory
response to the presence of intra-alveolar crystalline foreign material; the
foreign material was initially phagocytized by macrophages that then fused to
form large multinucleated cells.
1 IHC revealed that some of
the giant cells also contained intracytoplasmic MARV antigen.
The composition
of the crystalline foreign material, which is anisotropic in polarized light,
is unknown. However, a review of the medical records for this monkey revealed
that approximately one month before the initiation of the MARV study, this
animal had been administered an oral suspension of Pepcid® once a day for three
consecutive days. It is possible that some of the suspension was aspirated into
the right inferior lung lobe (which is a dependent lung lobe in a primate).
The active ingredient in the Pepcid® suspension is famotidine,
which is a crystalline compound, and inactive ingredients include
microcrystalline cellulose.
2 Overall, the foreign-body alveolitis
was a very mild and clinically insignificant lesion that did not affect the
pathogenesis or outcome of the MARV challenge. Anthracosilicosis is a common
finding in adult macaques and is usually
an incidental lesion (as in this case).
Note: Opinions,
interpretations, conclusions, and recommendations are those of the author and
are not necessarily endorsed by the U.S. Army.
JPC Diagnosis:
Lung: Alveolitis, histiocytic, multifocal, mild with low numbers
of multinucleated giant cell macrophages and abundant intracytoplasmic
crystalline protein, cynomolgus macaque,
Macaca fascicularis.
Conference Comment:
This
interesting case was submitted by the conference moderator and presented
participants with a diagnostic challenge to identify the origin of the
amphophilic, anisotropic, and crystalline material within macrophages and multi-nucleated
giant cells in this section of lung. Most favored the diagnosis of pneumo-coniosis,
which is a lung disease secondary to inhalation of inorganic particulate material,
such as asbestos or silica.
4 Readers are encouraged to review
Wednesday Slide Conference 2015 Conference
3 Case 4 for a review and fascinating discussion of silicate
pneumoconiosis in a horse from California. Silica dusts typically generate a
granulo-matous inflammatory response with fibrosis, not seen in this case.
Additionally, asbestos fibers in the lung are linear and beaded with globoid
ends, also not a feature of this case.
4
There have been sporadic
reports of kaolin aspiration in nonhuman primates causing similar lesions to
this case.
3,8,9 Kaolin is a common crystalline compound found in
antidiarrheal medication as well as a variety of other products, such as
toothpaste, ceramics, soap, and paint. Initially, the terminal bronchioles and
alveoli of animals exposed to aspirated or inhaled kaolin are acutely inflamed,
but by day seven post exposure, there is only mild mononuclear inflammation,
type II pneumocyte hyper-plasia, and aggregates of anisotropic dust-laden
macrophages and multinucleated cells.
9 This is in contrast to silica
inhalation, which induces a progressive granulomatous and fibrotic response.
4,9
The route of exposure of most reported cases in nonhuman primates is aspiration
of oral antidiarrheal medication.
8 Kaolin can also cause granulomas
containing numerous macrophages filled with birefringent crystals if delivered
subcutaneously.
3
To this authors knowledge,
there have been no reported cases of famotidine aspiration causing aspiration
alveolitis in humans or animals; although the pathogenesis posited by the
contributor is plausible. Unfortunately, given strict regulations on tissue
handling of Marburg (MARV)-infected animals, a tissue block was unable to be
submitted for further chemical analysis. Regardless of the origin of the
crystalline proteinaceous material, conference participants agreed that this
lesion is likely unrelated to MARV infection and is an incidental finding.
References:
1. Ackermann
MR. Inflammation and healing. In: Zachary JF and McGavin MD, ed.
Pathologic
Basis of Veterinary Disease. 5
th ed.
St Louis, Mo:
Elsevier, 2012:89-146.
2. Anonymous.
Pepcid oral suspension. Retrieved from: http://www.drugs.com/pro/pepcid-oral-suspension.html.
3. Baskin
GB.
Pathology of nonhuman primates. 1993. New Orleans: Tulane Regional
Primate Research Center.
4. Caswell JL, Willims KJ. Respiratory system. In: Maxie MG ed.
Jubb,
Kennedy, and Palmers pathology of domestic animals. Vol 2. 6
th
ed. St. Louis, Missouri: Elsevier; 2016:518.
5. Geisbert
TW, Hensley LE, Larsen T, et al. Pathogenesis of ebola hemorrhagic fever in
cynomologus macaques. Evidence that dendritic cells are early and sustained
targets of infection.
Am J Path. 2003; 163(6):2347-2370.
6. Geisbert
TW, Young HA, Jahrling PB, et al. Pathogenesis of ebola hemorrhagic fever in
primate models. Evidence that hemorrhage is not a direct effect of
virus-induced cytolysis of endothelial cells.
Am J Path. 2003; 163(6):
2371-2382.
7. Hensley
LE, Alves DA, Geisbert JB, et al. Pathogenesis of Marburg hemorrhagic fever in
cynomolgus macaques.
J Inf Dis. 2011; 204 (Suppl 3): S1021-S1031.
8. Herman
SJ, Olscamp GC, Weisbrod GL. Pulmonary kaolin granulomas.
J Can Assoc Radiol.
1982; 33(4):279-280.
9. Vallyathan
V, Schwegler D, et al. Comparative in vitro cytotoxicity and relative
pathogenicity of mineral dusts.
Ann Occup Hyg. 1988; 32:279-289