Wednesday Slide Conference, 2025-2026, Conference 11, Case 2
Signalment:
78-day-old Chicken (Gallus gallus)History:
Gross Pathology: The liver was enlarged and diffusely dark red. The spleen was moderately enlarged.Laboratory Results:
DNA was extracted from the liver homogenate. PCR for detecting avian leukosis virus (ALV) subgroup B and endogenous subgroup E was positive.Microscopic Description:
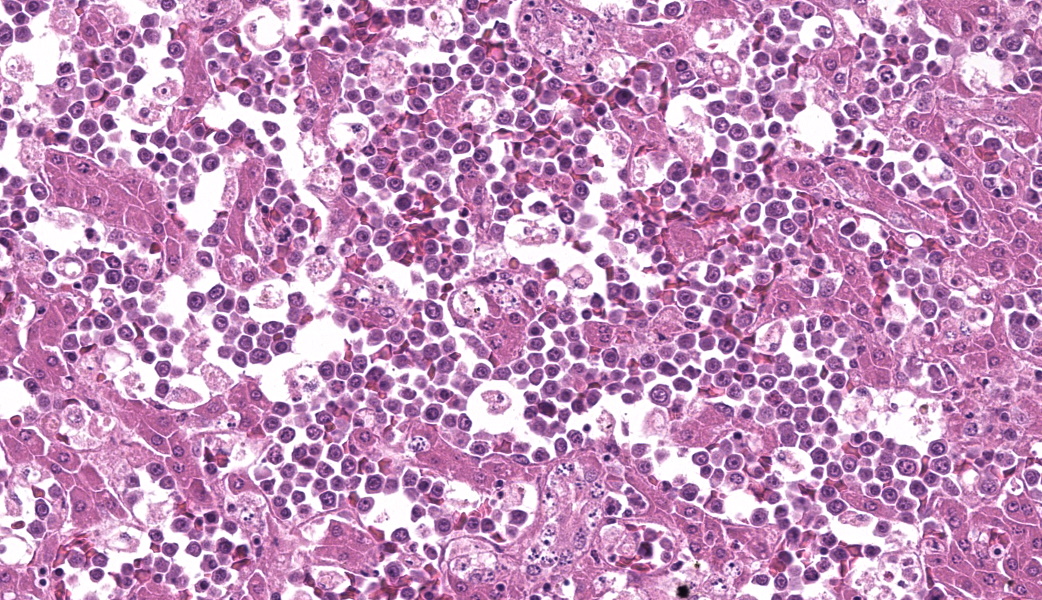
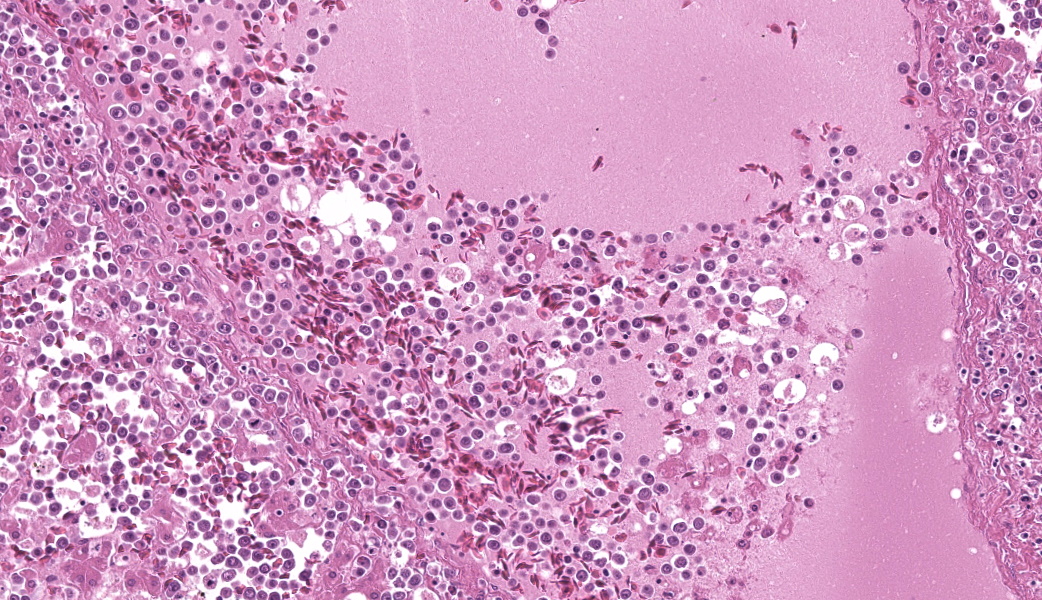
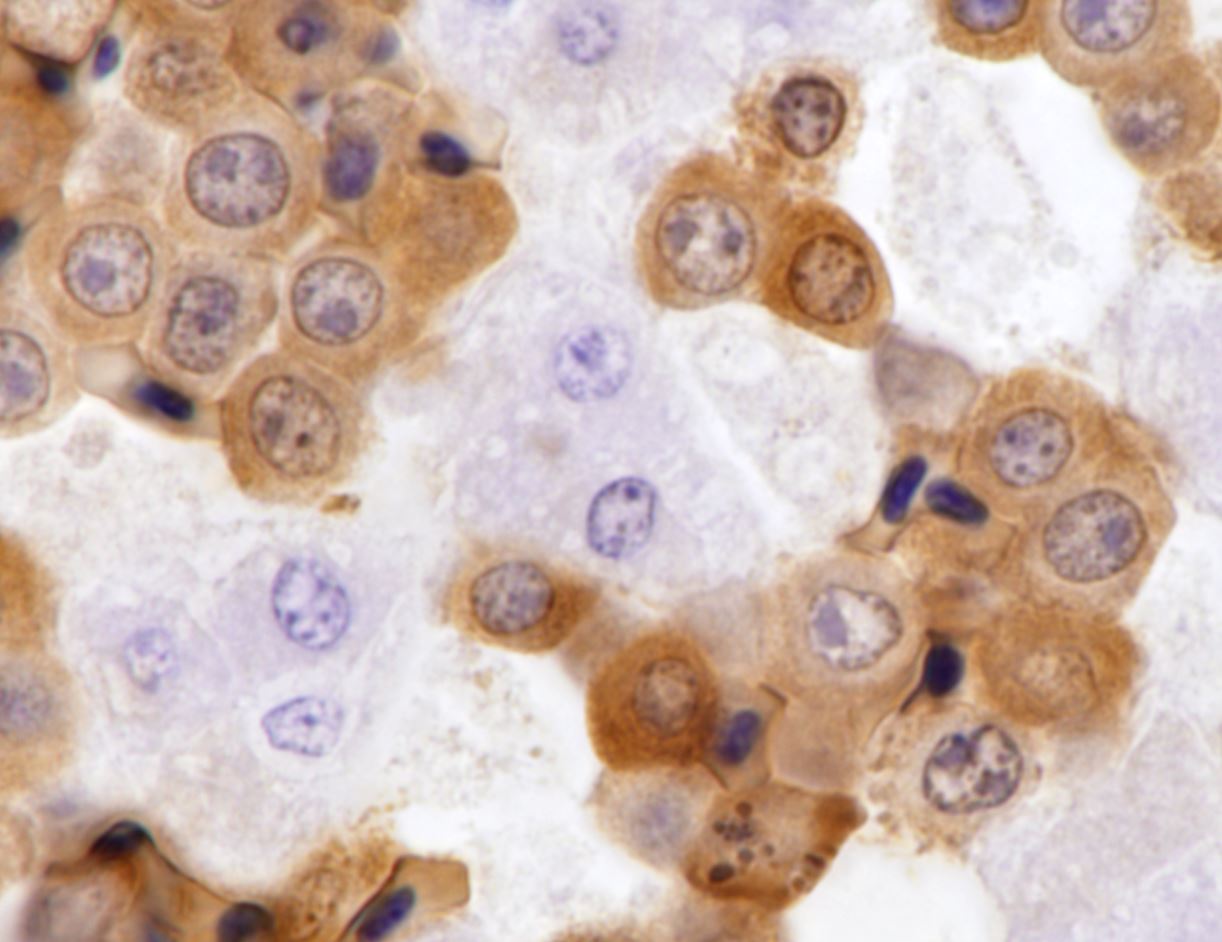
Round hematopoietic tumor cells strongly infiltrated the hepatic blood vessels and sinusoids. The hepatic sinusoids were diffusely expanded by tumor cell infiltration, with fewer normal erythrocytes and enlarged Kupffer cells which sometimes contained cellular debris. Most hepatocytes were shrunken. A few granulocytes were also found in the hepatic sinusoid. Small foci of granulocytic cells at different nuclear maturation stages were present around some hepatic triads. Tumor cells appeared round, oval, or polygonal in shape with a distinct cell border, amphophilic cytoplasm, anisocytosis, and anisokaryosis. Some tumor cells contained perinuclear pale areas known as halos. The nucleus was round and hyperchromatic to pale, with one or two large nucleoli and coarsely clumped chromatin. Some binuclear tumor cells were also observed. There was significant mitosis and karyorrhexis of tumor cells. The cytoplasmic eosinophilic granules that are characteristic to myelocytic lineage cells were not present in tumor cells.Immunohistochemical analysis revealed that tumor cells consistently exhibited cytoplasmic staining of hemoglobin antigen, with variable staining intensity, faint to strong, depending on the tumor cell. Hemoglobin was also detected in normal erythrocytes. Tumor cells were negative for CD3 (T cell marker), BAFF-R (B cell marker), and Iba-1 (macrophage/monocyte marker). Enlarged Kupffer cells were positive for Iba-1.
Tumor cells were observed in other organs, but only in the blood vessels. Some thrombi composed of necrotic tumor cells and fibrin were also observed in the vessels. The bone marrow was not histologically examined.
Contributor's Morphologic Diagnoses:
Avian erythroblastosisContributor's Comment:
Avian erythroblastosis (AE), also called erythroid leukosis, is a hematopoietic tumor of erythrocytic lineage cells in birds.2,7,9 Spontaneous occurrence of this tumor is rare in commercial chickens,2 although there are several experimental reports of AE in chickens inoculated with AE virus (AEV) and some other viral strains in the avian leukosis/sarcoma group.3,4,10,11,14,16,17 Experimentally, the viruses can induce AE and often other types of tumors, depending on the experimental conditions, such as the strain and dose of virus, inoculation route, and various host factors.3,4,10,11,14,16,17 Historically, AEV has been used in research on viral oncogene and erythrocytic differentiation.7,8,9 The relationship between the occurrence of AE and exogenous ALV infection is unclear in this case.On the basis of the histologic and IHC findings, the hematopoietic tumor was diagnosed as AE. The chicken exhibited findings suggestive of AE, such as an enlarged, cherry red liver, intravascular nature of tumor cells, and round hematopoietic tumor cells with occasional perinuclear halo.2,14 In addition, IHC detection of hemoglobin antigens in tumor cells was conclusive evidence of AE.
Hemoglobin is one of the markers useful for diagnosing erythrocytic tumors in humans and other mammals.6,15,18 Past studies have reported that hemoglobin is present in transformed AE cells of chickens inoculated with AEV.1,5 In past related studies conducting immunofluorescence assays of cultured AE cells transformed by AEV infection, the cells expressed a variety of avian erythrocytic markers, including hemoglobin, histone-5, erythroblast surface antigen, and erythrocyte surface antigen.3,8 A commercial rabbit monoclonal antibody (clone EPR3608) against the human hemoglobin can cross-react with chicken erythrocytes and erythrocytic precursors in the bone marrow on paraffin sections without nonspecific reactions.
Histologic differential diagnosis includes myeloid, lymphoid, and histiocytic tumors.2,13 In addition, a concurrent tumor of mixed AE and myelocytoma components has been described.2 A myeloid tumor can be differentiated from AE in that myeloid tumor cells replicate in both extra and intravascular space and often contain cytoplasmic granules.2,14 In our case, small foci consisting of granulocytic cells in the liver were considered by size, distribution, and cellular morphology as foci of extramedullary granulopoiesis, which are often found in avian tissue.2 Lymphoma was ruled out by negative IHC results by using anti-human CD3 and anti-chicken BAFF-R antibodies, which are the most appropriate commercial antibodies for detecting chicken T and B lymphocytes on FFPE sections.12 The negative IHC results with this anti-human Iba-1 antibody indicate that tumor cells in our case were not macrophage/monocyte lineage cells.
Contributing Institution:
National Institute of Animal Health, NARO.https://www.naro.go.jp/english/laboratory/niah/index.html
JPC Diagnoses:
- Liver: Intravascular round cell neoplasm.
- Liver: Extramedullary hematopoiesis, chronic, periportal, moderate.
JPC Comment:
This case proved to be challenging for participants given that the condition is relatively obscure and requires IHCs for definitive diagnosis. Leukemia and leukemic lymphoma were the top diagnoses considered by conference participants prior to case discussion. The contributor provides an excellent overview of and a convincing diagnostic workup for the diagnosis of avian erythroblastosis in their comment. An in-house hemoglobin IHC at the JPC similarly revealed moderate cytoplasmic immunoreactivity for hemoglobin within the neoplastic cell population and no immunoreactivity of neoplastic cells for lymphocyte markers (CD3, CD20, PAX5, CD34). This entity, and its accompanying histologic findings, provided great discussion of oncogenic viruses and intravascular round cell neoplasms of avian species. “Intravascular round cell neoplasm” was ultimately the morphologic diagnosis that was favored by the JPC due to the inability to reach the diagnosis of erythroblastosis on the H&E slide alone (the JPC morphologic diagnosis is always based on the HE section, which is the only section provided in advance of the conference.)Conference discussion focused largely on avian leukosis virus and its subtypes. Avian leukosis viruses (ALV) are alpharetroviruses that are usually slowly oncogenic. Subtype A is the most common, and subtypes A through E, as well as J, are known to infect chickens. Other subtypes affect numerous other species of birds. These viruses cause a variety of neoplasms by inserting themselves as promoters into the affected chicken’s genome near a cellular proto-oncogene, such as c-erbB, causing overexpression of the proto-oncogene. This overexpression leads to downstream dysregulation of cellular division and promotion of uncontrolled cell growth. Et voila, a neoplasm is produced! Such neoplasms include erythroblastosis, lymphomas (usually B-cell with IgM production), myelocytomatosis (subtype J), and various sarcomas.
Certain subtypes of ALV, such as subtype E, are endogenous to the DNA of chickens, where they live quietly and can be genetically inherited. These endogenous viral subtypes are considered to be non-oncogenic but can confound PCR results by producing false positives. As such, PCR for avian leukosis viruses is problematic and not generally reliable.
The subtypes of ALV that produce erythroblastosis, colloquially known as “avian erythroblastosis virus” (AEV), are unique among ALVs in that they stimulates rapid oncogenesis due to their genome containing its own potent oncogene (v-erbB).11,13 This oncogene is a mutated and shortened version of the c-erbB proto-oncogene within chicken DNA that is associated with other ALVs.13 The v-erbB oncogene results in the production of a mutated form of epidermal growth factor receptor (EGFR) protein, which is a type of tyrosine kinase receptor. This family of receptors is directly involved in cell signaling for growth and division. This receptor mutation results in loss of the ligand binding domain found in normal EGFR, as well as structural changes in its cytoplasmic regulatory regions. This enables the mutated EGFR to experience ligand-independent constitutive signaling activity and stimulate constant, transformative, cellular growth.13 As such, this virus is able to directly cause erythroblastosis and fibrosarcomas, which have a much more rapid onset of disease, whereas most ALVs cause slow-growing neoplasms via an indirect route.13 Chickens affected with avian erythroblastosis have a characteristically “cherry red” liver on gross examination.
Wrapping up the discussion of this fascinating and rare case was a quick review of other major oncogenic viruses in chickens, which included gallid herpesvirus-2 (causative agent of Marek’s disease, which usually affects young birds and results in a classical T-cell lymphoma) and avian gammaretrovirus (causative agent of avian reticuloendotheliosis).
References:
- Ambs E, Thorell B. On the type of hemoglobin in the neoplastic cell of virus-induced fowl erythroleukemia. J Natl Cancer Inst. 1960;25:685–695.
- Barnes HJ, Fletcher OJ. Hemic system. In: Abdul-Aziz T, Fletcher OJ, Barnes HJ, eds. Avian histopathology. 4th ed. Jacksonville, FL: American Association of Avian Pathologists. 2016:1–16.
- Beug H, von Kirchbach A, D¨oderlein G, et al. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 1979:18:375–390.
- Burmester BR, Adrian Gross M, Walter WG, et al. Pathogenicity of a viral strain (RPL12) causing avian visceral lymphomatosis and related neoplasms. II. Host-virus interrelations affecting response. J Natl Cancer Inst. 1959;22:103–127.
- Cowles J, Saikkonen J, Thorell B. On the presence of hemoglobin in erythroleukemia cells. Blood 1958;13:1176–1184.
- Edamoto H, Suwa K, Tamura K. Spontaneous erythroid leukemia in a 6-wk-old male Crlj: B6C3F1 mouse. J Toxicol Pathol. 2017;20:101–104.
- Graf T, Beug H. Avian leukemia viruses interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978;516:269–299.
- Graf T, Beug H, Royer-Pokora B, et al. In vitro transformation of hematopoietic cells by avian erythroid and myeloid leukemia viruses: a model system for the differentiation of normal and neoplastic cells. In: Clarkson B, Marks P, Till JE, eds. Differentiation of normal and neoplastic hematopoietic cells. New York, NY: Cold Spring Harbor Laboratory. 1978:625–639.
- Hayman MJ, Beug H. Avian erythroblastosis: a model system to study oncogene co-operation in leukemia. Cancer Surv. 1992;15:53–68.
- Hihara H, Yammamoto H, Ishino S, et al. Pathogenicity of some clones from field isolates of avian leukosis viruses. Natl Inst Anim Health Q. 1972;12:117–126.
- Hihara H, Yamamoto H, Shimohira H, et al. Avian erythroblastosis virus isolated from chick erythroblastosis induced by lymphatic leukemia virus subgroup A. J Natl Cancer Inst. 1983;70:891–897.
- Kurokawa A, Yamamoto Y. Immunohistochemical identification of T and B lymphocytes in formalin-fixed paraffin-embedded chicken tissues using commercial antibodies. Vet Immunol Immunopathol. 2020;228:110088.
- Massoglia S, Gray A, Dull TJ, Munemitsu S, Kun HJ, Schlessinger J, Ullrich A. Epidermal growth factor receptor cytoplasmic domain mutations trigger ligand-independent transformation. Mol Cell Biol. 1990;10(6):3048-55.
- Nair V. Leukosis/sarcoma group. In: Swayne DE, Boulianne M, Logue C, et al., eds. Diseases of poultry,14th ed. Hoboken, NJ: Wiley-Blackwell. 2020:587–625.
- Ogasawara F, Kumagai Y, Mikami O, et al. Erythroblastic sarcoma in the thoracic cavity of a cow. J Vet Med Sci. 2019;81:134–137.
- Purchase HG. The pathogenesis and pathology of neoplasms caused by avian leukosis viruses. In: de Boer GF, ed., Avian leukosis. Boston, MA: Martinus Nijhoff Publishing. 1986:171–196.
- Venugopal K, Howes K, Flannery DM, et al. Isolation of acutely transforming subgroup J avian leukosis viruses that induce erythroblastosis and myelocytomatosis. Avian Pathol. 2000;29:327–332.
- Wang W, Wang SA, Medeiros LJ, et al. Pure erythroid leukemia. Am J Hematol. 2017;92:292–296.