Signalment:
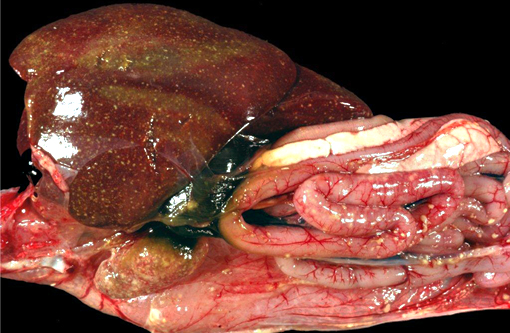
Gross Description:
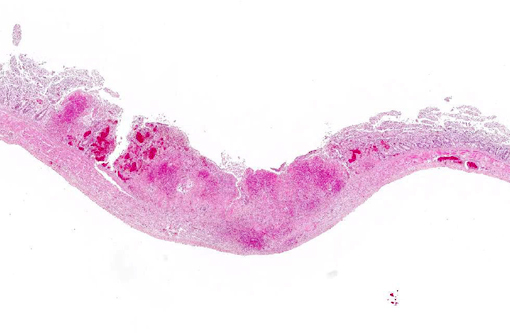
Histopathologic Description:
Morphologic Diagnosis:
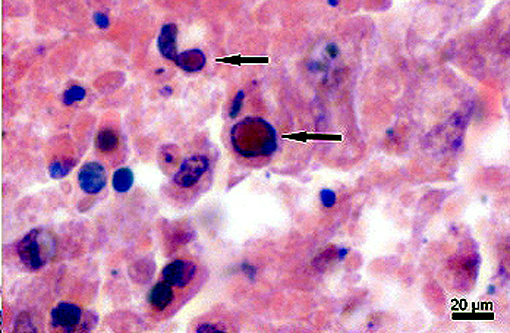
Microscopic Findings of Tissues not submitted: In the liver and spleen multifocal areas of acute necrosis with intralesional eosinophilic intranuclear inclusion bodies are present.
Condition:
Contributor Comment:
Sequence analyses revealed an almost 100% sequence identity between FHV-1, CoHV-1, and StHV-1 whereas there were considerable differences to other avian herpesviruses.(8) These results further supported the hypothesis that owls and falcons might get infected by the consumption of pigeons infected with CoHV-1.(6,8) Natural infections with StHV-1 have been observed in the eagle owl (Bubo bubo L.), long-eared owl (Asio otus L.) and snowy owl (Bubo scandiacus) as well as in great horned owls (Bubo virginianus) in the United States. (2,8)
The majority of infected owls are found dead without previous clinical signs. However, some show depression, anorexia, conjunctivitis, oral and pharyngeal ulcerations and respiratory symptoms as well as diarrhea.(2,6)
The main histologic feature of the disease is the presence of numerous necrotic foci in the liver, spleen and bone marrow.(6) Typically, in the liver, spleen and several additional organs, eosinophilic intranuclear inclusion bodies of Cowdry A type are present. Necrotic areas may appear in the pharynx and small intestine as well.(6)
JPC Diagnosis:
Conference Comment:
Herpesviruses are well-known, ubiquitous infectious agents across the entire animal kingdom, with new strains being discovered regularly, often which pose significant health threats to the affected population. Recent publications have described herpesviral necrotizing stomatitis in Eastern box turtles (terrapene herpesvirus-1),(12) significant mortality in koi and common carp (cyprinid herpesvirus-3),(5) and a sarcoma inducing herpesvirus in baboons (Kaposis Sarcoma-associated herpesvirus).(13) Adding further interest to the subject are the peculiarities of pathogenesis and disease transmission among some herpes pathogens, such as the seasonal-dependent neoplastic growth or viral shedding of ranid herpesvirus-1 in leopard frogs(3) and the recent proposal of chelonid herpesvirus-5 superspreaders causing outbreaks of fibropapillomatosis in marine turtles.(14)
Central to the success of all herpesviruses is the interplay between latency and lytic modes of infection. Latency is characterized by restricted viral gene expression permitting the virus to evade the host immune system. Upon reactivation, a cascade of gene expression is initiated enabling its spread between cells and between hosts. In all subfamilies of human herpesviruses, the latent to lytic switch has been found to occur through expression of one or more microRNAs (miRNA).(4) MiRNAs are noncoding, or transcribed but not translated, single stranded RNAs which inhibit translation of messenger RNA through the action of the RNA-induced silencing complex (RISC). In this way, they are able to control cell growth, differentiation and survival. Their characteristics are now well understood regarding development of cancer and their expression is conserved among all eukaryotes, including microorganisms such has the numerous herpesviruses discussed here.(9)
References:
1. B+�-+rki F, Burtscher H, Sibalin M: Herpesvirus strigis: a new avian herpesvirus. I. Biological properties. Arch Gesamte Virusforsch. 43: 14-24, 1973.
2. Burtscher H, Sibalin M: Herpesvirus strigis: host spectrum and distribution in infected owls. J Wildl Dis. 11: 164-9, 1975.
3. Carlson DL, Sauerbier W, Rollins-Smith LA, McKinnell RG. The presence of DNA sequences of the Lucke herpesvirus in normal and neoplastic kidney tissue of Rana pipiens. J Comp Pathol. 1994;110:349-355.
4. Frapper L. Regulation of herpesvirus reactivation by host miRNAs. J Virol. 2014 Dec 24 pii: JVI.03413-14 [Epub ahead of print].
5. Fujioka H, Yamasaki K, Furusawa K. Prevalence and characteristics of Cyprinid herpesvirus 3 (CyHV-3) infection in common carp (Cyprinus carpio L.) inhabiting three rivers in Kochi Prefecture, Japan. Vet Microbiol. 2014 Dec 11. Pii: S0378-1135(14)005574 [Epub ahead of print]
6. Gailbreath KL, Oaks JL: Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (columbid herpesvirus 1). J Wildl Dis. 44: 427-33, 2008.
7. Green RG, Schillinger JE: A virus disease of owls. Am J Pathol. 12: 405-410, 1936.
8. Kaleta EF: Herpesviruses of birds - a review. Avian Pathol. 19: 193-211, 1990
9. Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2015:320.
10. Potgieter LN, Kocan AA, Kocan KM; Isolation of a herpesvirus from an American kestrel with inclusion body disease. J Wildl Dis. 15: 143-9, 1979.
11. Rose N, Warren AL, Whiteside D, et. al. Columbid herpesvirus-1 mortality in great horned owls (Bubo virginianus) from Calgary, Alberta. Can Vet J. 2012;53(3):265-268.
12. Sim RR, Norton TM, Bronson E, et. al. Identification of a novel herpesvirus in captive Eastern box turtles (Terrapene carolina carolina). Vet Microbiol. 2014 Dec 13. Pii: S0378-1135(14)005549. [Epub ahead of print]
13. Whitby D, Stossel A, Gamache C, et. al. Novel Kaposis sarcoma-associated herpesvirus homolog in baboons. J Virol. 2003;77(14):8159-8165.
14. Work TM, Dagenais J, Balazs GH, Schettle N, Ackermann M. Dynamics of virus shedding and in situ confirmation of chelonid herpesvirus 5 in Hawaiian green turtles with fibropapillomatosis. Vet Pathol. 2014 Dec 1 DOI: 10.1177/03000985814560236 [Epub ahead of print].