Signalment:
Six-year-old female French Alpine goat, (
Capra aegagrus hircus).This goat was
suspected to have aborted prior to necropsy, although no expelled fetus was
found. This goat also aborted last year, but had successfully kidded in the past.
Gross Description:
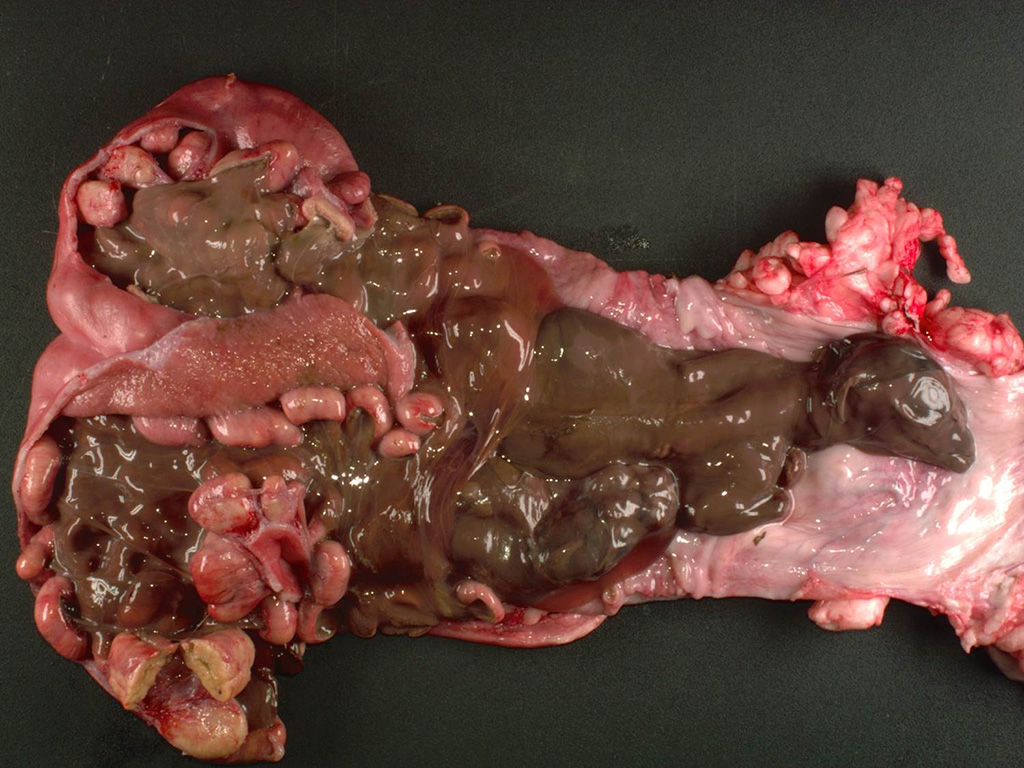
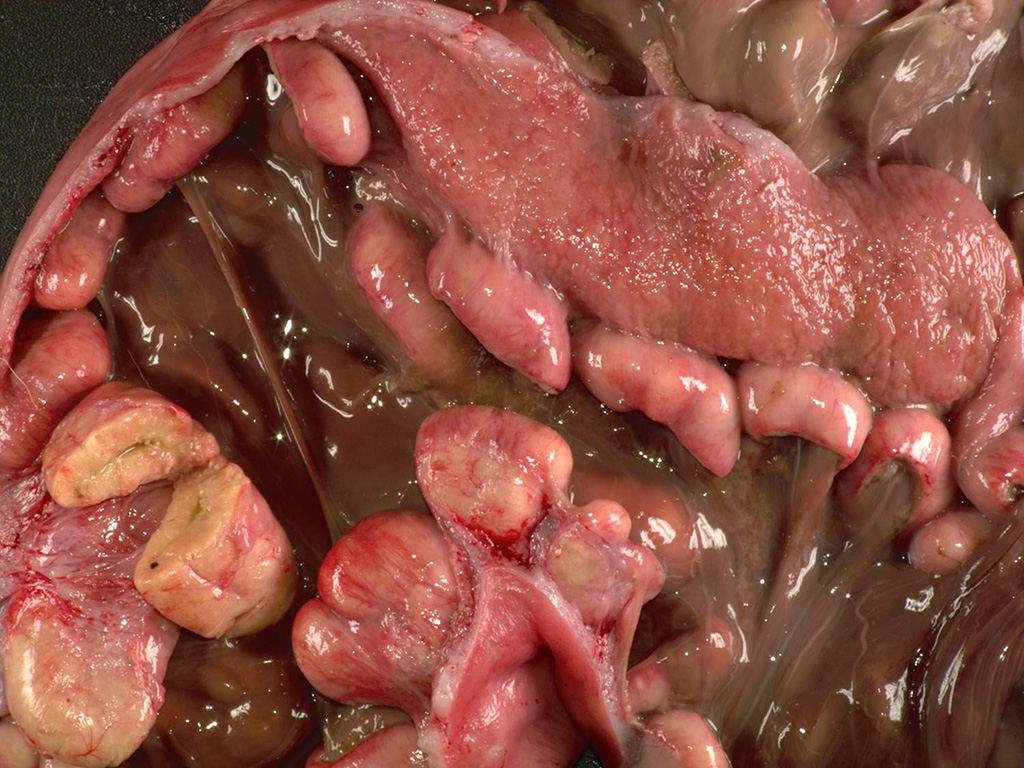
The
uterine body contained two macerated fetuses with a crown to rump length of 16
cm and 10 cm. Within the uterus, all of the caruncles were enlarged (~4x2x1
cm), homogeneous, and pale tan.
Histopathologic Description:
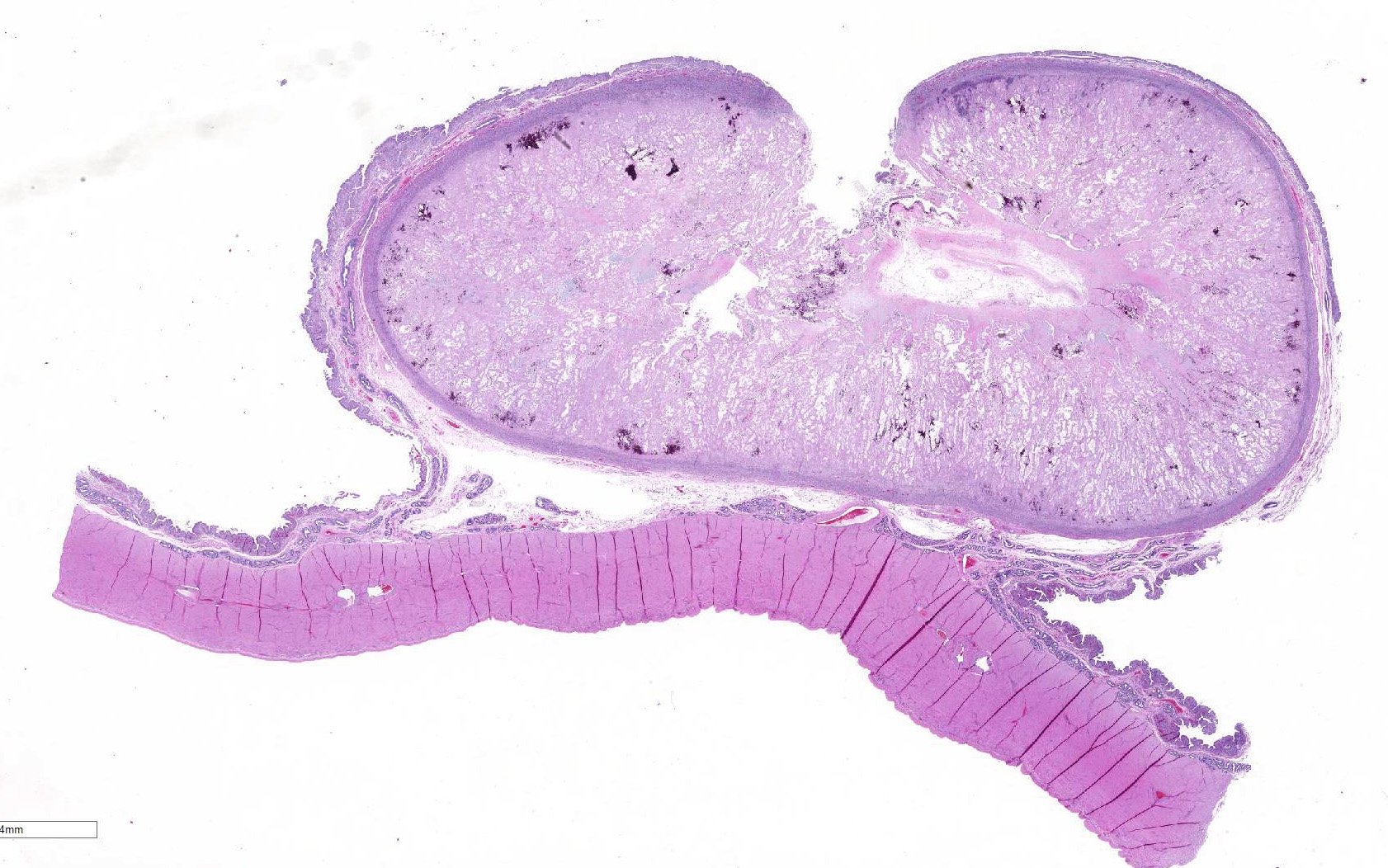
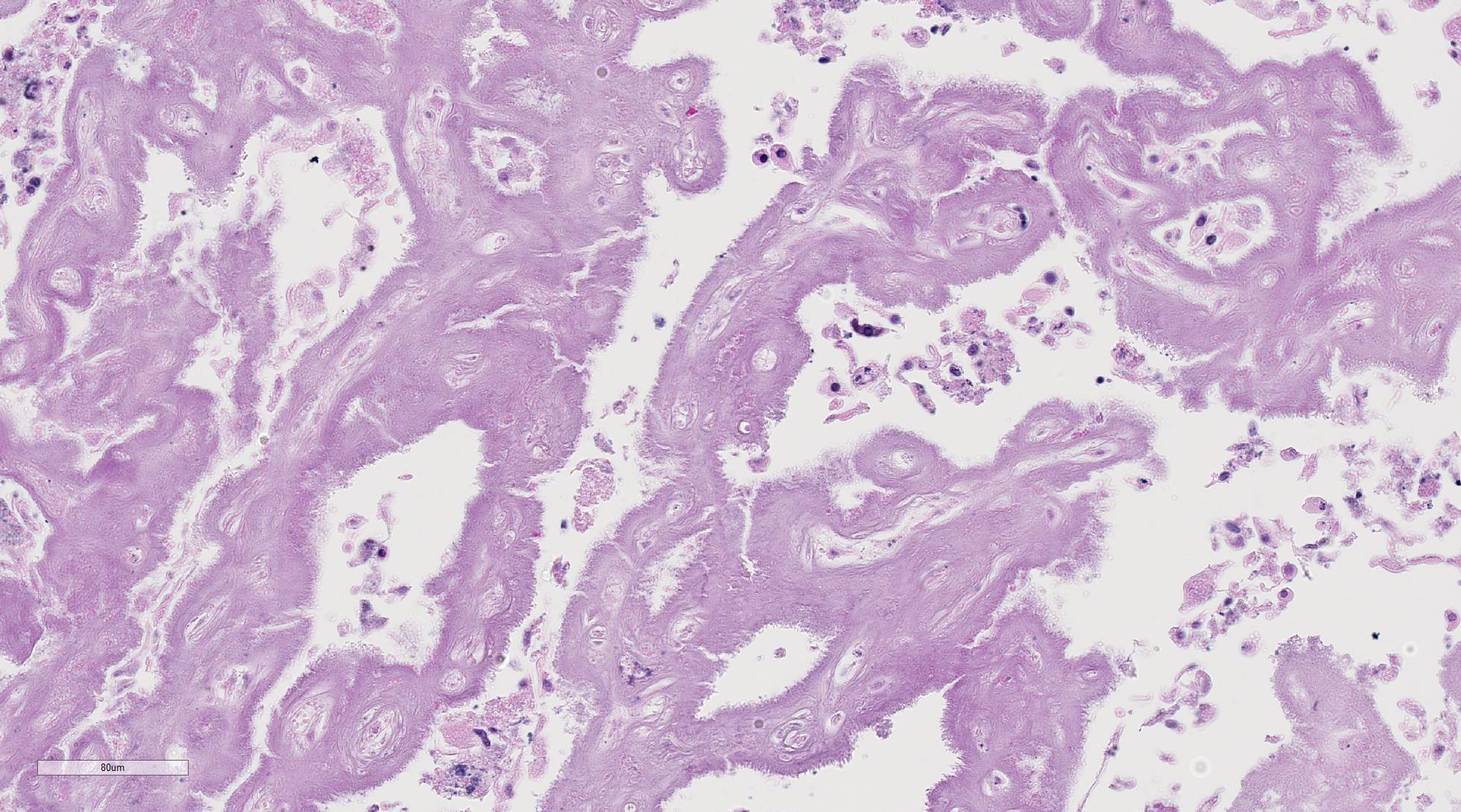
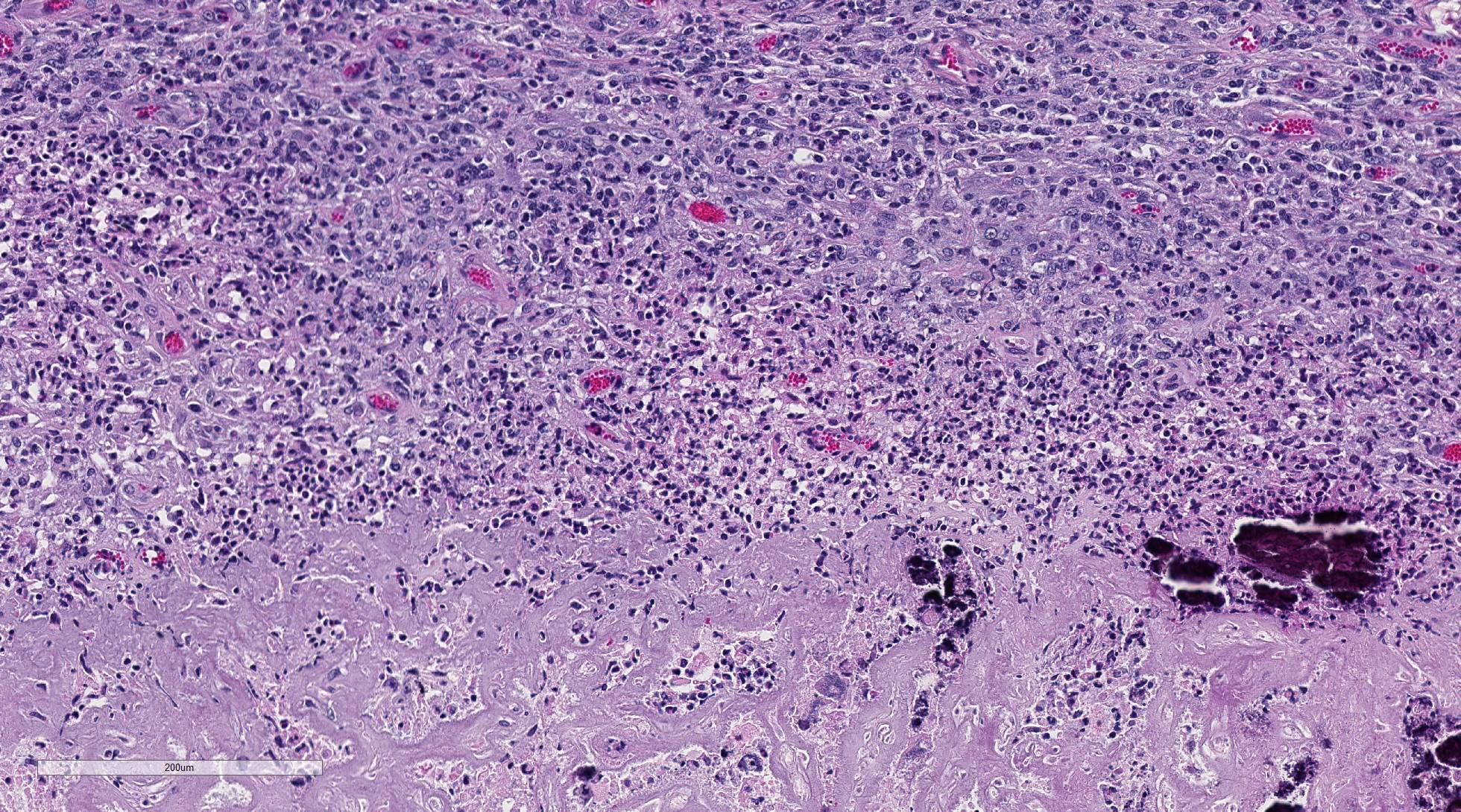
The
uterine caruncular labyrinth is diffusely expanded by abundant pale
eosinophilic homogenous, extracellular material which is multifocally disrupted
by areas of blue granular mineralization. The interdigitating cotyledonary
villi are sparse, and the allantoic stroma is mildly expanded by edema. The
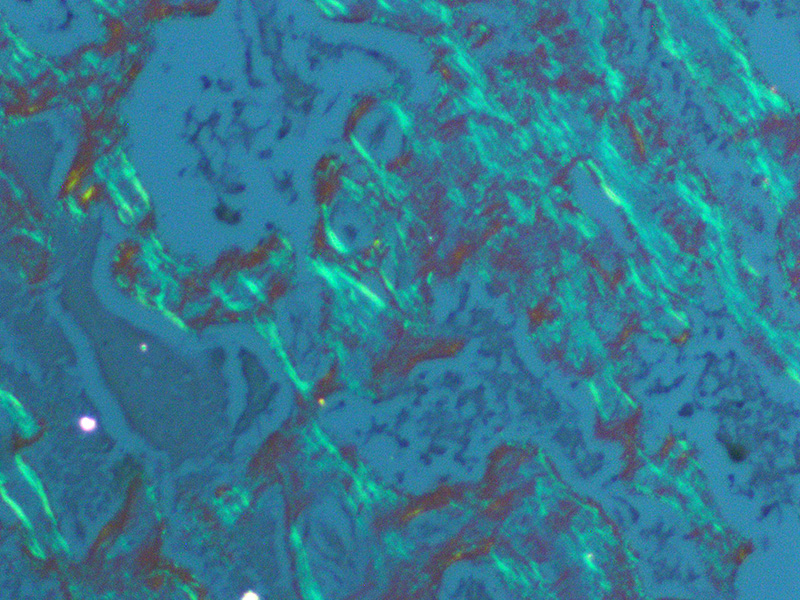
aforementioned interstitial eosinophilic material within the caruncles stains
orange/pink with Congo red and exhibits apple green birefringence with
polarized light, consistent with amyloid. The umbilicated surface of the
placentome is multifocally ulcerated and replaced by large aggregates of
neutrophils, lymphocytes, and histiocytes. Similar inflammatory cells extend
into the subepithelial stroma of the caruncle, endometrium, and minimally
throughout the labyrinth. The placental and endometrial stroma is expanded by
moderate amounts of edema, few scattered inflammatory cells, and multifocal
aggregates of mineral. There are also multifocal areas of mineralization
throughout the tunica media of medium-sized vessels within the placenta and
endometrium.
Morphologic Diagnosis:
1. Uterus: Diffuse interstitial caruncular amyloid
2. Uterus and
placenta: Chronic necrotizing placentitis and endometritis with mineralization
Lab Results:
Bacterial culture and sensitivity (uterus):
Numerous
Escherichia coli
Few
Enterococcus faecalis
No
growth of
Brucella species
Chlamydophila
sp
, PCR (uterus): Negative
Coxiella
burnetii, PCR (uterus): Negative
Condition:
Bronchointerstitial pneumonia/Influenza virus
Contributor Comment:
Caruncular
amyloidosis has been previously reported in a small number of goats in
California. Clinical presentation of such goats included mid-to-late term
abortion that often occurred repeatedly over multiple years which was attributed
to impaired gas exchange at the site of fetal attachment.
2 Ages
ranged from 3-8, and breeds included Toggenburg, La Mancha, and Saan. Similar
to the California goats, this goat had no evidence of amyloid deposition in
other organs nor was there evidence of a systemic or chronic disease process.
Few bacteria were isolated from the inflamed region of the placentome but are
considered to be secondary to the retained fetuses. Bacterial culture did not
isolate
Brucella abortus, and PCR was negative for
Chlamidophila
species and
Coxiella burnetti.
In general,
amyloid is composed of insoluble aggregates of misfolded proteins, and
deposition of amyloid can occur in a wide variety of localized or systemic
diseases.
8 Although the fibrillar component of amyloid is overall
similar in composition, a diverse number of proteins with variation in sequence
and structure are considered amyloidogenic.
7,8 Common amyloid
precursors include: serum amyloid A (SAA), amyloid
light chain (AL), islet amyloid polypeptide (IAPP), mutated forms of
transthyretin, and beta protein amyloid.
7,8
In
particular, SAA proteins comprise a family of apolipoproteins that can be
synthesized hepatically and/or extrahepatically. Hepatic derived SAA (SAA1 and
SAA2) can dramatically increase in response to inflammation. In mice, rats,
cows, and rabbits SAA3 appears to be the most common extrahepatic SAA in
addition to being produced hepatically.
3 Increased production of
SAA3 has also been described in bovine and human mammary gland epithelium in
response to prolactin, and in uterine papillary cancer. In the goats in
California increased levels of SAA3 were detected within the endometrium when
compared to the liver, suggesting localized expression. The type and cause of
the amyloid deposition in this case is currently unknown, but the localized
caruncular involvement is similar to what has been previously described and may
represent a new syndrome of goats.
2
JPC Diagnosis:
Placenta, caruncle: Amyloidosis, diffuse, marked, French
Alpine goat,
Capra aegagrus hircus
Conference Comment:
The contributor provides an informative summary of the
pathogenesis of amyloidosis and review of previously reported cases of a unique
syndrome of caruncular amyloidosis causing abortion in goats. This excellent
case confounded conference participants on initial examination of the tissue
section. Virtually every attendee interpreted the amorphous, smudgy, homogenous
eosinophilic material that diffusely expands the uterine lattice as a
geographic area of coagulative necrosis admixed with multifocal mineralization
and mild inflammatory infiltrate in the subepithelial stroma of the caruncle
and endometrium; collectively, the findings were attributed to normal post
kidding involutional change, rather than caruncular amyloidosis. Similar to the
findings reported by the contributor, Congo red histochemical staining of
serial sections performed at the JPC demonstrates the eosinophilic
proteinaceous material is diffusely congophilic and displays bright apple-green
birefringence when viewed under polarized light.
Spirited
discussion ensued among conference participants regarding the presence of
concurrent diffuse necrosis, autolysis, or a combination of both admixed with
the deposited amyloid in the tissue section. Most favored diffuse necrosis of
both the epithelial and endothelial cells secondary to amyloid deposition, resulting in infarction of the
placentome. Discord over the presence or absence of necrosis or autolysis
nothwithstanding, this case nicely demonstrates a newly reported syndrome of
reproductive failure in goats secondary to uterine amyloid deposition in the
endometrium at the site of placental attachment.
2 Accumulation of
amyloid within the carucle markedly compromises blood flow and both gas and
nutrient exchange
between the doe and the fetus; this leads to fetal hypoxia and eventually
death.
2 Similar to previously reported cases of caruncular
amyloidosis in goats
2, amyloid is not present within the
intercaruncular endometrium, myometrium, endometrial glands, or vessels in this
doe.
Before discussing this case, participants reviewed the normal
placentation in small ruminants. All ruminants have similar cotyledonary
placentation composed of the fetal cotyledon and the maternal caruncle. The
placenta contains the maternal endometrium and the fetal chorioallantoic
membranes (CAM).
1 Ruminant placentas are nondeciduate, indicating
that the maternal endometrium and fetal CAM are in close contact, but they do
not intimately fuse. In cotyledonary placentation, there are numerous areas
where the fetal cotyledon villar attachments interdigitate with the crypts of
the caruncular epithelium. The combination of the fetal cotyledon and maternal
caruncle make up the placentome.
1 In sheep and goats, the caruncles
have a characteristic concave shape, nicely demonstrated in this case.
1
Bovine placentomes are similar in structure and function, but are convex rather
than concave.
Conference
participants discussed various causes of abortion in small ruminants, to
include infectious agents such as
Chlamydophila abortus,
Toxoplasma
gondii,
Brucella ovis,
Campylobacter fetus,
Coxiella
burnetii, and
Listeria monocytogenes. Non-infectious causes include
plant toxins, such as locoweed poisoning, and nutritional factors including
dietary deficiencies of copper, magnesium, vitamin A, and selenium.
5,6
After reviewing this case, conference participants agreed that caruncular
amyloidosis should be considered as an additional differential diagnosis of
non-infectious abortion in the goat.
References:
1. Bacha WJ, Bacha LM.
Color Atlas of Veterinary
Histology. 3
rd ed. Baltimore,
MD: Lippincott Williams & Wilkins; 2012:243-260.
2. Gaffney PM, Barr
B, Rowe JD, Bett C, Drygiannakis I, Giannitti F, Trejo M, Ghassemian M, Martin
P, Masliah E, Sigurdson C. Protein profiling of isolated uterine AA Amyloidosis
causing fetal death in goats. FASEB J. 2015; 29:911-919.
3. Larson MA, Wei
SH, Weber A, Weber AT, McDonald TL. Induction of human mammary associated serum
amyloid A3 expression by prolactin or lipopolysaccharide. Biochem Biophys
Res Commun. 2003; 301:10301037.
4. OBrien
TD, Butler PC, Westermark P, Johnson KH. Islet amyloid polypeptide: A review
of its biology and potential roles in the pathogenesis of diabetes mellitus. Vet
Pathol. 1993; 30: 317-332.
5. Sanad
YM, Jung K, Kashoma I, et al. Insights into potential pathogenesis mechanisms
associated with Campylobacter
jejuni-induced abortions in ewes. BMC
Vet Res. 2014; 10:274-287.
6. Schlafer
DH and Foster RA. Diseases of the gravid uterus, placenta and fetus In: Maxie
MG, ed. Jubb Kennedy and
Palmer's Pathology of Domestic Animals.
Vol 3. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:407-408.
7. Tani Y, Uchida
S, Nakamura H, Nakayama N, Goto D. Amyloid deposits in the gastrointestinal
tract of aging dogs. Vet Pathol. 1997; 34:415-420.
8. Teoh SL, Griffin
MD, Howlett GJ. Apolipoprotein and amyloid fibril formation in atherosclerosis.
Protein Cell. 2011; 2(2):116-127.