Signalment:
Four-year-old, male, red tail boa constrictor, (
Boa constrictor constrictor).The
patient initially presented on 10/29/201 4 for a 4-5 month history of anorexia.
At that time, there was atrophy of the epaxial muscles and a firm, but
compressible swelling expanding the cranial cervical region. Ultrasound of this
area revealed a soft-tissue mass, for which the tissue of origin was unclear.
The mass did not appear to be associated with the trachea or within the esophageal
lumen. The patient represented in January 2015 for continued anorexia,
progressive lethargy, and recent regurgitation after forced feeding. The
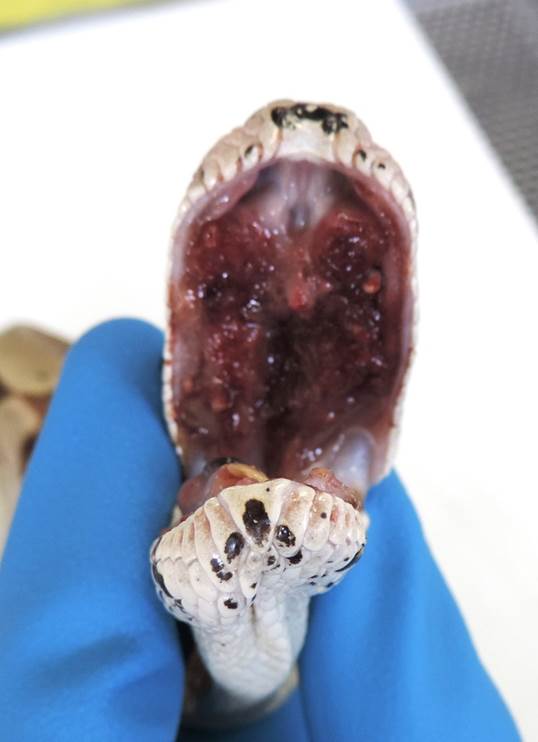
patient had lost a significant amount of weight and on examination, there was a
large accumulation of necrotic material in the mouth. During this exam, a
second mass was appreciated just caudal to the previously described cervical
mass. At the owner's request, the patient was euthanized.
Gross Description:
A 2 cm x 0.5 cm region of the hard palate is raised,
irregularly surfaced, fleshy, and dark red. Extending from the esophageal wall
and protruding into the lumen is a 2.5 cm x 1 cm x 1 cm, smooth, soft, pink and
red mass, with a core of crumbly, brown-yellow material. Approximately 0.5 cm
aboral to this mass is a 4.5 cm x 2.5 cm x 2.5 cm, smooth, firm, ovoid, pink
and red mass that on section is composed of a central core of a crumbly,
brown-yellow material (caseous necrosis) surrounded by a 0.7 cm wide rim of a
fleshy, pink/red tissue. The corresponding eso-phageal serosa is firmly adhered
to the local body wall. The esophageal mucosa, adjacent to the larger mass, has
a 2 cm x 0.4 cm, poorly demarcated, mildly depressed, region composed of dozens
of pinpoint red foci (erosion). Within the lumen of the aboral esophagus,
approximately 3.5 cm from the larger mass, are three rough, ovoid aggregates of
a mottled brown, yellow, and green, crumbly material. This material is not
adhered to the mucosa.
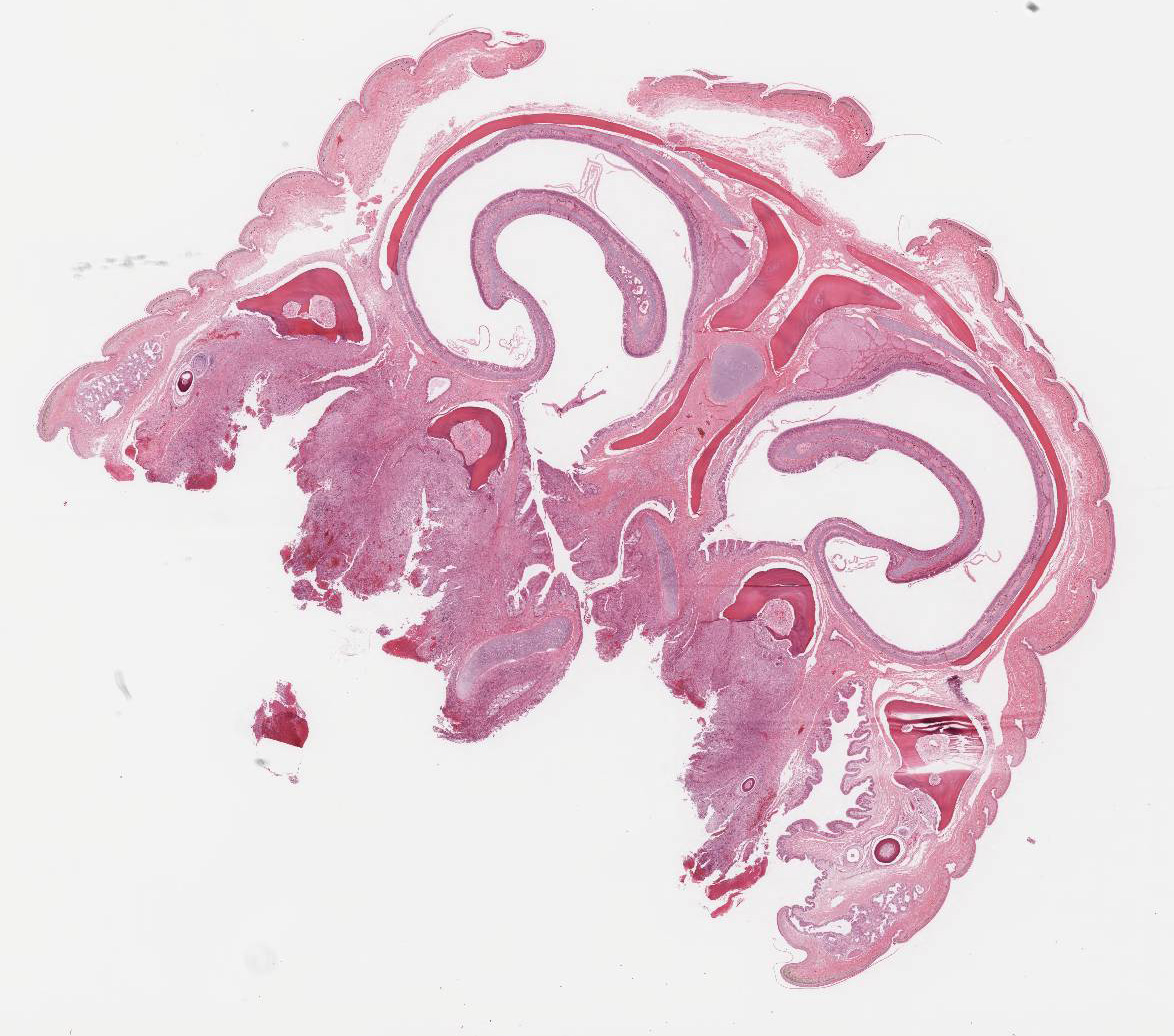
Histopathologic Description:
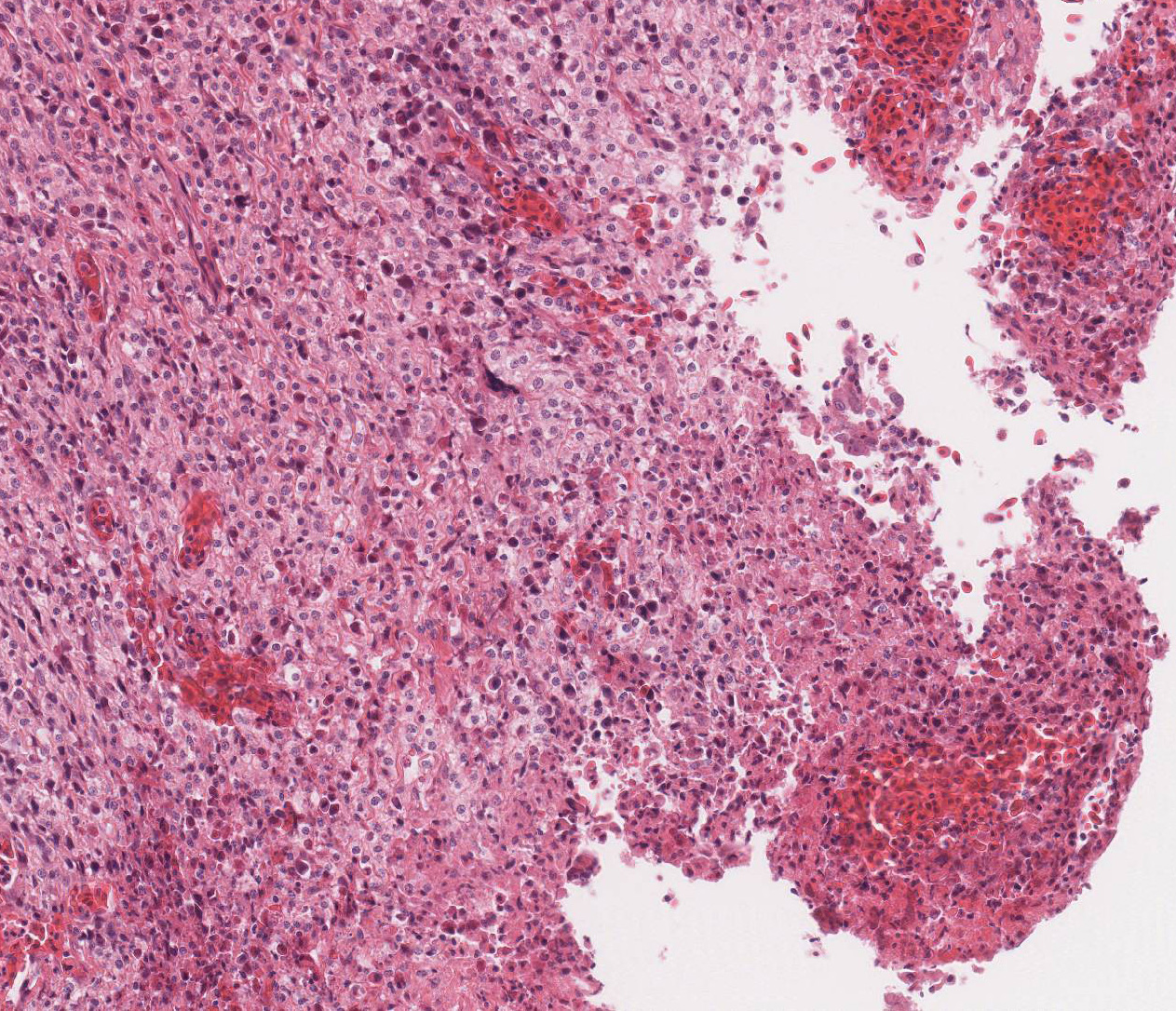
ORAL CAVITY: Severely expanding the sub-mucosa, confluent
with a large region of epithelial ulceration, and obliterating the adjacent
lamellar bone is a densely packed population of foamy macrophages with fewer
granulocytes, lymphocytes, and plasma cells. Unilaterally the alveolar bone is
disrupted and replaced by a similar inflammatory cell population and the
islands of retained bone has irregular, scalloped edges and are lined by
numerous osteoclasts within distinct Howship's lacunae. The superficial osteoid
matrix commonly contains a thin, irregular, basophilic line (reversal line).
Similar inflammatory cells efface the dentin and invade the pulp of a tooth.
The surface epithelium is entirely replaced by a band of necrotic cellular
debris and fibrin.
BRAIN,
NOS: Occasional neurons contain discrete, intracytoplasmic, 1-10 um diameter,
glassy, eosinophilic inclusions.
NASAL
CARTILAGE: Commonly, the respiratory epithelial cells and submucosal gland
epithelial cells contain discrete, intracytoplasmic, 1 -1 0 um diameter,
glassy, eosinophilic inclusions. Scattered throughout the submucosa are scant
numbers of lymphocytes and plasma cells.
ESOPHAGEAL
MASSES (not submitted): Multifocally the esophageal wall is severely expanded
by multiple unencapsulated, irregular masses composed of densely packed
vacuolated macrophages mixed with fewer small lymphocytes, plasma cells,
granulocytes, and multinucleated nucleated giant cells. Lymphocytes are
occasionally present in small, poorly defined islands and cells contain
discrete, intracytoplasmic, 1-4 um diameter, glassy, eosinophilic inclusions.
The overlying epithelial cells contain discrete, 2-6 um, glassy, eosinophilic,
intra-cytoplasmic inclusions.
Additional findings include numerous discrete, 1-10um in
diameter, glassy, eosinophilic, intracytoplasmic inclusions within multiple
tissues including hepato-cytes, biliary epithelial cells, gastric mucosa,
intestinal epithelium, tracheal epithelium, bronchiole epithelium, and the
retinal ganglion cells. Concurrently within the liver, there were small numbers
of randomly distributed macrophages, lymphocytes, and plasma cells.
Morphologic Diagnosis:
Oral cavity: Severe, diffuse,
chronic, granulomatous and ulcerative stomatitis with numerous, eosinophilic,
intracytoplasmic inclusion bodies
Esophageal
mass (not submitted): Severe, multifocal, chronic, granulomatous and ulcerative
esophagitis with numerous, eosinophilic, intracytoplasmic inclusion bodies
Stomach,
intestine, trachea, lung, kidney, liver, nasal cavity, retina, and brain (not
submitted): Severe, eosinophilic, intra-cytoplasmic inclusion bodies
Lab Results:
N/A
Condition:
Boid inclusion disease, stomatitis
Contributor Comment:
Inclusion body disease (IBD) is reported in multiple snake
species, but most commonly within the family
Boidae and
Pythonidae.
As the name implies, the characteristic finding in these cases are distinct,
variably sized, eosinophilic, intracytoplasmic inclusions.
2 In one
recent retrospective study, the prevalence of IBD in captive collections was
approximately 19%. Although disease progression varies greatly between
individuals and between species, the disease is classically associated with
central nervous system signs, including head tremors, anisocoria, and
opisthotonus.
9 Commonly the animal succumbs to complications
secondary to immunosuppression.
15 In boas, the disease can have a
more protracted progression of weeks to months
9 and typically
patients have a previous history of regurgitation.
10 In addition, a
proportion of boas can be subclinical carriers.
7-10 Meanwhile,
pythons tend to display a more aggressive disease course of only a few weeks,
9
with a more profound inflammatory reaction.
10 Interestingly,
regurgitation tends not to be a part of clinical disease in pythons.
10
Gold standard testing remains the histologic demonstration of intracytoplasmic
inclusions in multiple organs, most notably the liver, stomach, and esophageal
tonsils, however, their absence does not rule out the disease.
10
Although with further characterization of the underlying etiology, molecular testing and immunohisto-chemistry
3 may
be available in the future. Although
long considered to have an underlying infectious etiology, the cause of IBD has
been elusive. Originally the disease was thought to be associated with a
retrovirus, however, recently divergent arenaviruses have been implicated as
the cause of IBD.
2-4,8-10 Of late,
in vitro Koch's postulates
have been met linking arenavirus to the development of IBD, however, in vivo
studies have not be reported.
9 Arenaviridae are enveloped, negative
sense, single stranded, bipartite RNA viruses.
2,8 Arenaviruses have
previously been thought to only affect rodents, with infrequent but possible
transmission to other mammal species (e.g. humans and bats).
2 The
viral genome is composed of a small (S) segment and a large (L) segment. The S
segment encodes the viral nucleocapsid protein (NP) and the glycoproteins (GP1
and GP2) while the L segment encodes the viral RNA-dependent RNA polymerase and
a small ring domain containing protein
2,9 The distinction
intracytoplasmic inclusions consist of a unique 68KDa protein, that has been
named "inclusion body disease protein" (IBDP).
3,9,10 This
protein has been demonstrated to be the arenaviral NP protein.
6
Boid-associated inclusion body arenaviruses tend to be highly divergent.
2,8,9
Proposed
theories as to the mode of transmission include direct contact, possible
arachnid vectors (i.e. the snake mite,
Ophionyssus natricis), mammalian
vectors (i.e. live prey), and vertical transmission.
8-10 As several
other arenaviruses are able to cross the species barrier, it remains possible
that the highly divergent boid inclusion body disease associated arenaviruses
(BIBDAV) may as well. A recent report showed that BIBDAV was infective to tick
cells lines (mite cell cultures were not available) and this data may support
the role of Ophionyssus
natricis infestation in disease transmission.
8
Furthermore, maintenance of BIBDAV within mammalian (VERO E6) and boid cell
lines appears to be temperature dependent, with strong growth at 30°C and
inhibition of growth at 37°C. Thus, the authors purpose that transmission from
a mammalian host is possibly hindered by the higher mammalian body temperature.
8Boid
snakes have large well-developed esophageal tonsils, which can be enlarged and
abscessed. In this case, the large esophageal masses may represent severely
inflamed esophageal tonsils.
JPC Diagnosis:
1.Oral cavity: Stomatitis, ulcerative and histiocytic, chronic,
multifocal to coalescing, severe, with granulation tissue and bone resorption,
red tail boa constrictor,
Boa constrictor constrictor. Epithelial cells: epidermis, salivary gland, and nasal cavity: Intracytoplasmic
protein inclusions of viral origin, numerous.
Conference Comment:
Boid
inclusion body disease (BIBD) is considered by many to be the most important
viral infection of captive boas and pythons, often causing progressive and
rapidly fatal multisystemic disease.
10 As mentioned by the
contributor, the clinical presentation of BIBD is highly variable among
individuals, especially in adult boas, where intracytoplasmic viral protein
inclusions can be found in snakes without clinical signs.
2,6,10,13 However,
the disease is still considered to be fatal due to severe impairment of immune
function of leukocytes and myelopoietic cells, resulting in death due to
opportunistic infections.
6,10 Interestingly, in this case, the
conference moderator speculates that the extensive ulcerative stomatitis seen
both grossly and histologically may be due to BIBD-induced starvation and
regurgitation combined with the mechanical trauma from the reported forced
feeding.
Typical gross findings
associated with BIBD are usually limited to areas susceptible to secondary
opportunistic infections, such as the oral cavity, gastrointestinal tract,
lungs, liver, and kidney. Histologically, the hallmark of BIBD is the presence
of numerous 1-4 um, pale, eosinophilic intra-cytoplasmic inclusion bodies in
all major organs, especially the kidneys, liver, stomach, and brain. Inclusions
are typically more prominent in the visceral organs of boas and central nervous
system in pythons.
10 In this case, inclusions are widely distributed
throughout.
Conference participants
discussed the composition of the highly distinctive inclusion bodies associated
with this disease. The inclusions, which ultrastructurally are
cytoplasmic aggregates of electron-dense material, composed of a n
antigenically unique 68-kilodalton non-viral protein.
2,3,6,15
Recently, a novel group of arenaviruses were isolated from snakes with BIBD. In
an in-vitro cell culture model, this arenavirus induces the pathognomonic
inclusion bodies and was discovered to predominantly consist of arenaviral
associated nuclear protein. This finding led to the suggestion of the formation
of a novel genus called the
Reptarenavirus and placing the remaining
arenaviruses in the
Mammarenavirus genus. There is still some
controversy surrounding the formation of a new genus given the lack of
in
vivo confirmation.
3,9,11
Conference participants
discussed the importance of arenaviruses as zoonotic pathogens associated with
rodent host species. In humans, arenaviruses, such as Lassa and Machupo
(Bolivian hemorrhagic fever), cause outbreaks of rapidly fatal viral
hemorrhagic fevers, not unlike the Ebolavirus.
1 Additionally,
hamsters are the primary source of lymphocytic chorio-meningitis (LCM) virus
causing meningo-encephalitis in humans and callitrichid hepatitis in New World
primates.
13 Arenaviruses generally produce only mild or subclinical
disease in their natural host species.
References:
1. Bell
TM, Bunton TE, Shaia CI, Raymond JW, Honnold SP, Donnelly GC, Shamblin JD,
Wilkinson ER, Cashman KA. Pathogenesis of Bolivian hemorrhagic fever in Guinea
pigs.
Vet Pathol. 2016; 53(1):190-199.
2. Bodewes, R, Kik, MJL, Raj S, et al. Detection of novel
divergent arenaviruses in boid snakes with inclusion body disease in the
Netherlands.
J Gen Virol. 2013; 94:1206-1210.
3. Chang, L, Fu Am Wozniak, E, et al. lmmunohistochemical
detection of a unique protein within cells of snakes having inclusion body
disease, a world-wide disease seen in members of the families
boidae and pythonidea.
PLoS One. 2013. 8(12):1-16.
4. Charrel RN, de Lamballerie X. Zoonotic aspects of arenavirus
infections.
Vet Microbiol. 2010; 140:21320.
5. Drake, S, Marschang RE, Hetzel U, et al. Experimental
infection of boa constrictor with an orthoreovirus isolated from a snake with
inclusion body disease.
J Zoo Wildl Med. 2014; 45(2):433-436.
6. Fowler ME, Miller RE.
Fowler's Zoo and Wild Animal
Medicine. Miller RE, Fowler ME eds. Vol 8. Philadelphia, PA; Saunders;
2014:70.
7. Hellebuyck, T, Pasmans F, Ducatelle, R, et al. Detection of
arenavirus in a peripheral ondotogenic fibromyxoma in a red tail boa (
Boa
constrictor constrictor) with inclusion body disease.
J Vet Diagn Invest.
2015; 27(2):245-248.
8. Hepojoki J, Kipar, A, Korzyukov, et al. Replication of boid
inclusion body disease-associated arenaviruses is temperature sensitive in both
boid and mammalian cells.
J Virol. 2015; 89(2):1119-1128.
9. Hetzel U, Sironen T, Laurinmaki, P, et al. Isolation,
identification, and characterization of novel arenaviruses the etiological
agents of boid inclusion body disease.
J Virol. 2013; 87(20):10918-10935.
10. Jacobson
ER.
Infectious Diseases and Pathology of Reptiles, Color Atlas and Text.
Jacobson ER ed.1
st ed. CRC Press, Boca Raton, 2007:185, 410-412.
11. Keller
S, Hetzel U, et al. Co-infecting reptarenaviruses can be vertically transmitted
in boa constrictor.
PLoS Pathog. 2017; 13(1):e1006197.
12. Pees
M, Schmidt V, Marschang RE, et al. Prevalence of viral infections in captive
collection of boid snakes in Germany.
Vet Rec. 2010; 166:422-425.
13. Montali RJ,
Connolly BM, Armstrong DL, Scanga CA, Holmes KV: Pathology and immunology of
callitrichid hepatitis, an emerging disease of captive new world primates
caused by lymphocytic choriomeningitis virus.
Am J Path. 1995; 148(5):144-149.
14. Schillinger
L, Selleri P, Frye FL. Lymphoblastic lymphoma and leukemia blood profile in a
red-tail boa (
Boa constrictor constrictor) with concurrent inclusion
body disease. J
Vet Diag Invest. 2011; 23:159-162.
15. Schmidt
V, Marschang RE, Abbas MD, et al. Detection of pathogens in boidae and
pythonidae with and without respiratory disease.
Vet Rec. 2013;
172(9):236.