CASE I: 20N-0086 (JPC 4153158)
Signalment:
9-year-old, spayed female, Otterhound (Canis familiaris), canine
History:
The dog had an approximately 3-week history of right forelimb lameness with a swollen, warm, painful elbow joint that was nonresponsive to nonsteroidal anti-inflammatory drugs. Bilateral elbow radiographs showed a right fragmented medial coronoid process with mild degenerative joint disease. A complete blood count was performed and revealed a mild thrombocytosis (651 x 103/μL), leukocytosis (15.6 x 103/μL), and neutrophilia (13.3 x 103/μL) and a presumptive diagnosis of septic arthritis was made. The night before presentation to necropsy, the owner reported an increased respiratory rate and effort. The following morning, the dog was found deceased.
Gross Pathology:
Gross examination revealed diffuse generalized muscle wasting. The lungs were diffusely heavy. Scattered throughout all lung lobes were pinpoint to 2 mm in diameter tan to red to dark red foci that extended into the parenchyma on cut surface. On cut surface, the lungs exuded a moderate amount of dark red watery to foamy fluid. The trachea contained a moderate amount of tan to red foamy fluid.
Lateral to the right elbow joint, there was a multicavitated structure that exuded a mild amount of tan watery fluid and extended caudally and proximally encircling the olecranon and dissecting through the surrounding musculature. This structure did not directly communicate with the elbow
joint but did infiltrate the synovium externally. The olecranon also had multifocal areas of boney lysis and the proximal tip was markedly friable. There was a fragment on the proximal-medial surface of the ulna that was similar in color and texture to the adjacent bone (fragmented medial coronoid process). There was also mild roughening and thinning of the articular cartilage in the right elbow joint (degenerative joint disease). The synovial fluid in the joint was clear with the viscosity of saliva.
Laboratory Results:
4DX SNAP: Negative x 4
Bilateral elbow radiographs revealed a right fragmented medial coronoid process with mild degenerative joint disease. The left elbow was within normal limits.
Synovial fluid analysis revealed a markedly increased protein concentration; however, interpretation was inconclusive due to hemodiluted sample.
Aerobic and anaerobic culture of the right joint demonstrated no growth after 7 days.
CBC: mild thrombocytosis (651 x 10^3/ μL), leukocytosis (15.6 x 10^3/ μL), and neutrophilia (13.3 x 10^3/ μL)
Serum chemistry: Within normal limits
Microscopic Description:
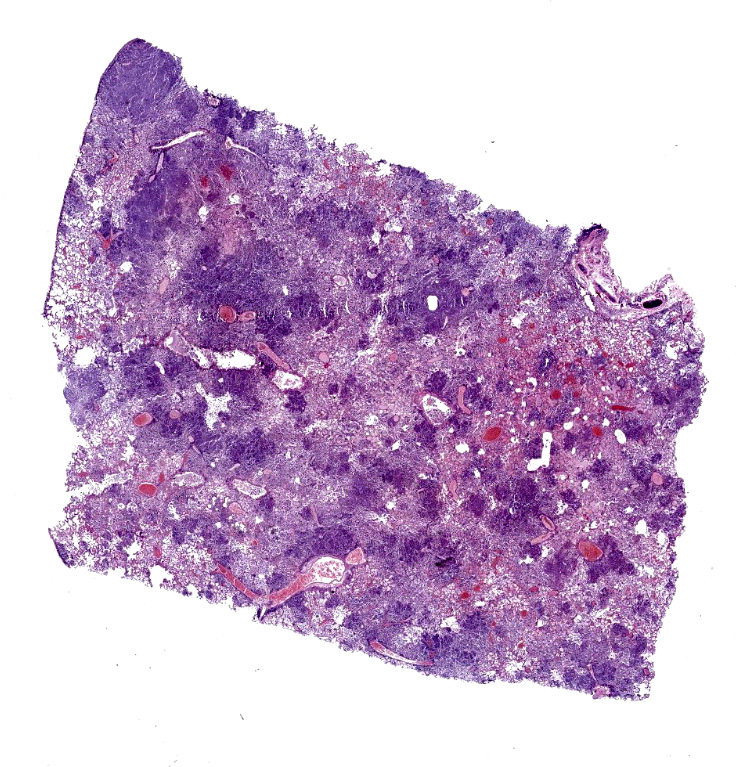
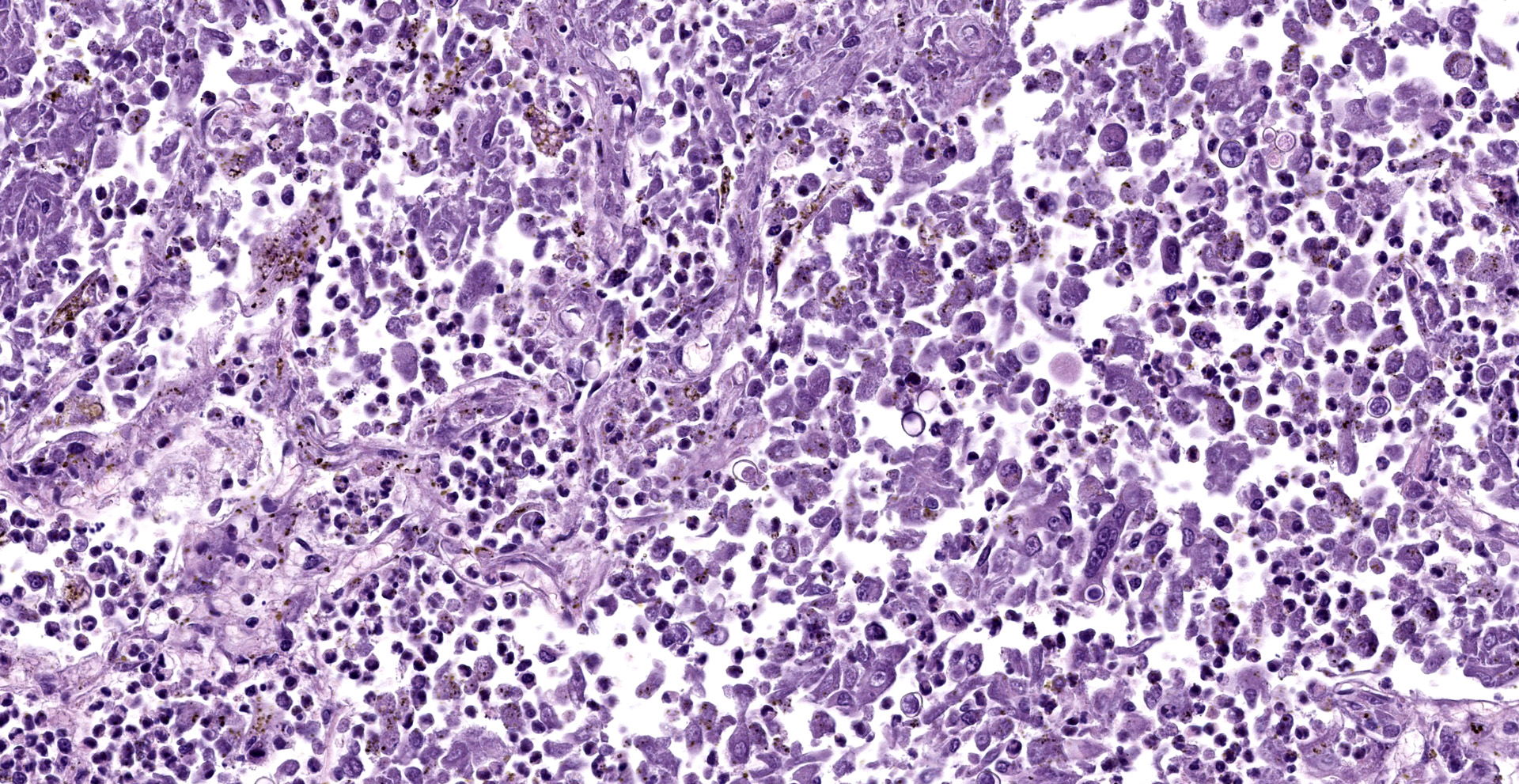
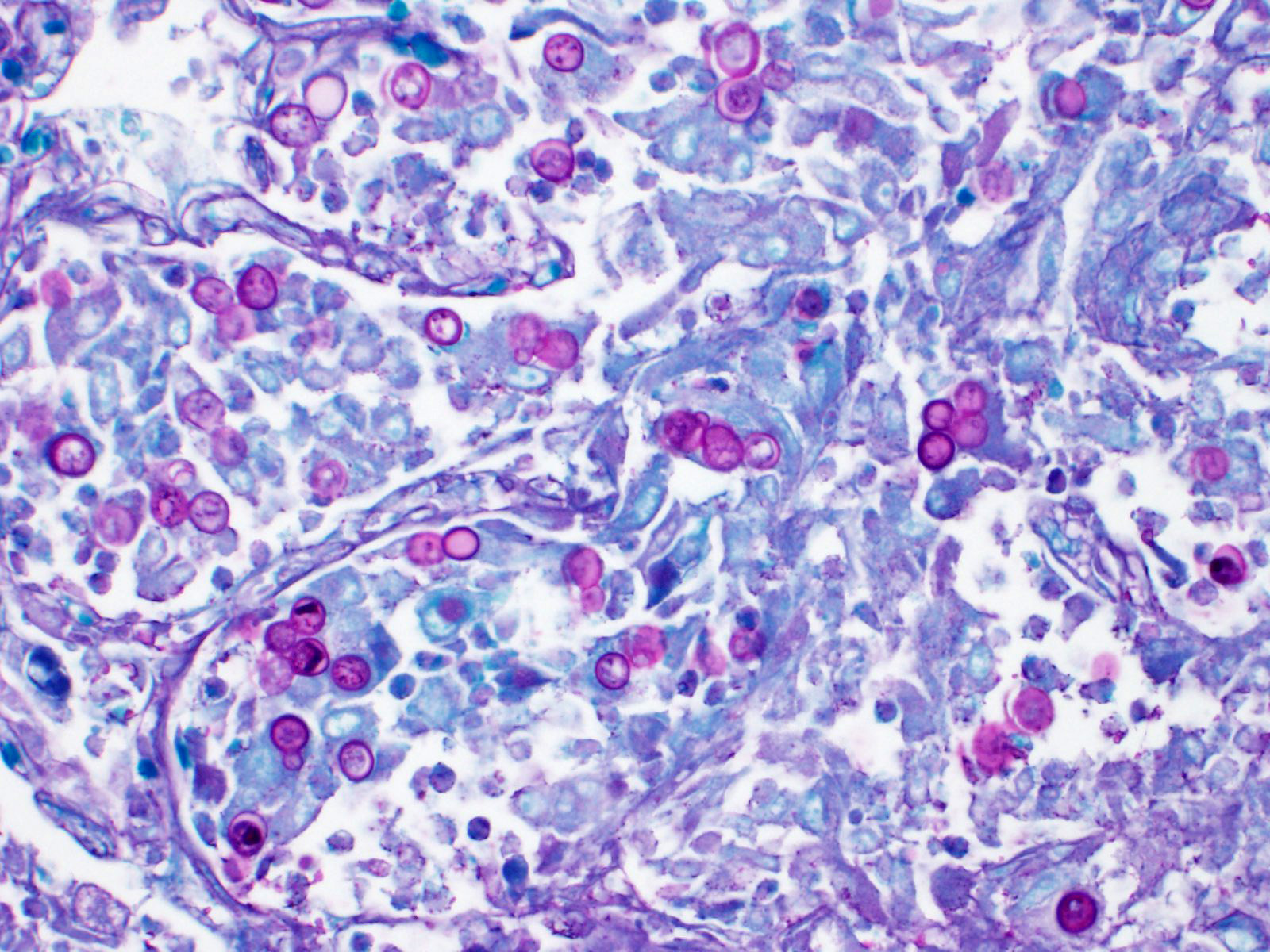
Lung: Affecting approximately 50 to 90% of the examined sections, there is marked infiltration, expansion, and replacement of alveoli, terminal bronchioles, and less often alveolar septa by a densely cellular inflammatory infiltrate. Inflammatory cells are composed predominately of foamy and epithelioid macrophages and viable and degenerative neutrophils with fewer multinucleated giant cells (foreign body and Langhans types), lymphocytes and plasma cells. Inflammatory cells form disorganized loose aggregates that expand nearly all alveoli and bronchioles to varying degrees but also occasionally form poorly organized pyogranulomas that are variably surrounded by a thin wall of fibrous connective tissue. In the most severely inflamed regions, there is multifocal to coalescing coagulative necrosis with retention of cellular architecture but loss of differential staining, which affects approximately 20%-60% of the tissue.
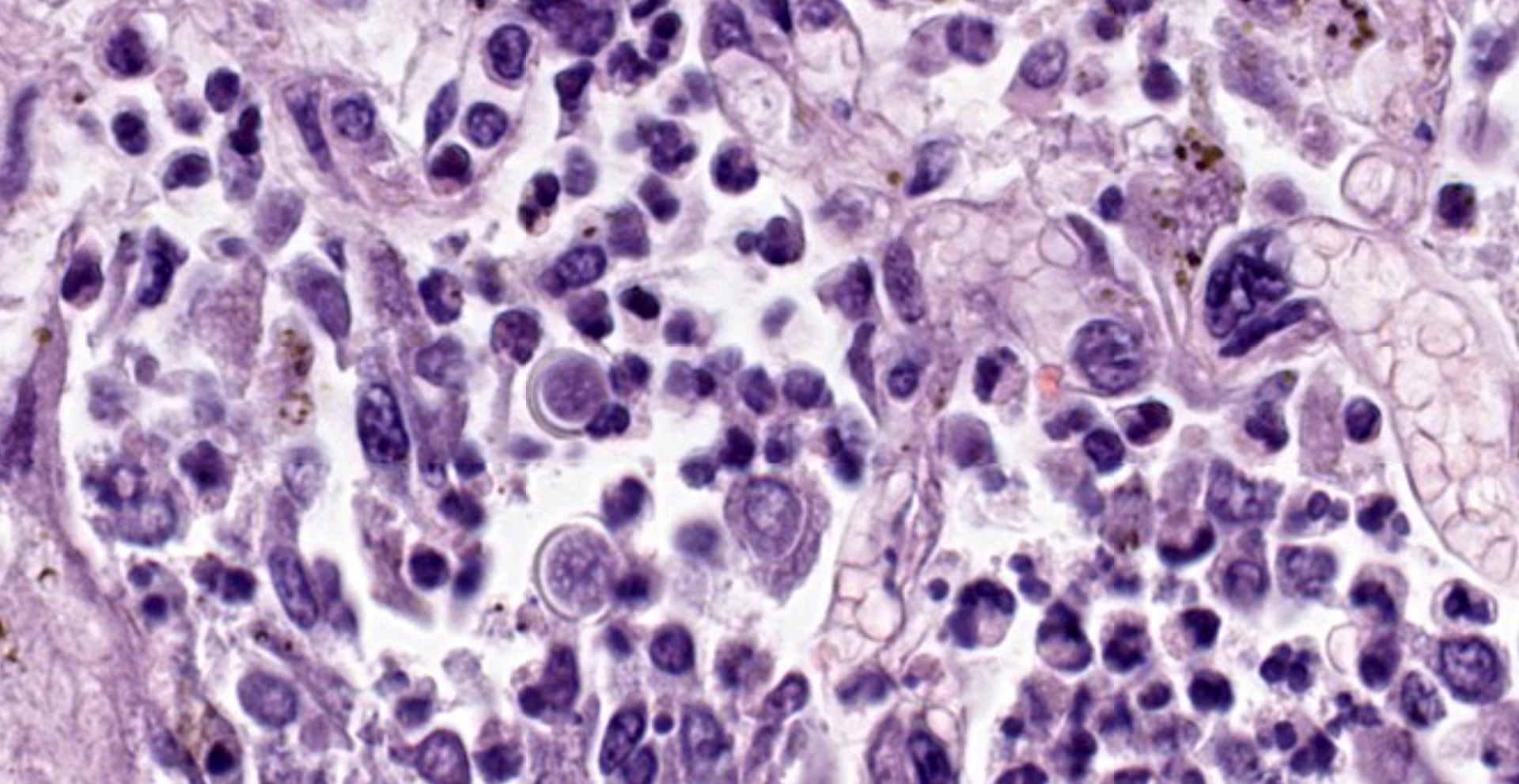
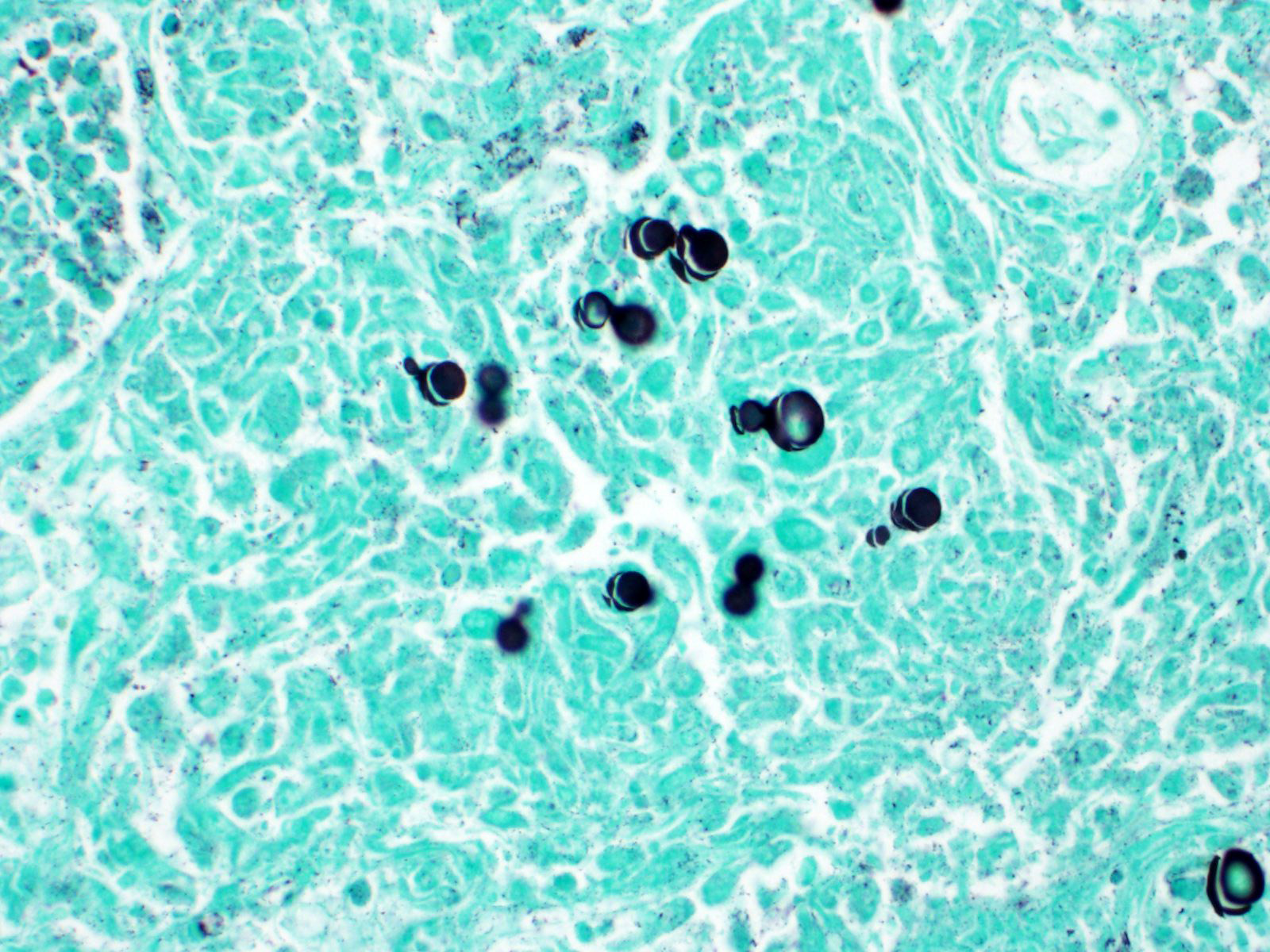
Within areas of inflammation and necrosis, there are numerous extracellular and intrahistiocytic (including multinucleate giant cells), 5-30 μm in diameter, round yeast with a 1-2 μm distinct double-contoured refractile wall. They typically lack nuclear material / protoplasm and rarely exhibit broad-based budding. In less affected areas, alveoli contain fibrillar material (fibrin), hemorrhage, eosinophilic proteinaceous edema fluid and mildly increased numbers of foamy macrophages and neutrophils. Vessels are diffusely congested and variably lined by hypertrophied endothelial cells. Large caliber bronchioles and bronchi are largely unaffected but do contain a small amount of sloughed epithelial cells (postmortem autolysis) and erythrocytes, and the submucosal connective tissue is infiltrated by individual to loosely arranged small clusters of lymphocytes and plasma cells with fewer macrophages and neutrophils.
Tissue from around the right elbow (not submitted) is affected by pyogranulomatous inflammation containing numerous yeasts similar to those described in the lung.
Contributor's Morphologic Diagnoses:
Lungs: marked, multifocal to coalescing, pyogranulomatous interstitial pneumonia with myriad intrahistiocytic and extracellular broad-based budding yeasts consistent with Blastomyces dermatitidis (pulmonary blastomycosis)
Contributor's Comment:
The histologic changes in this case represent a classic presentation of pulmonary blastomysosis caused by Blastomyces dermatitidis. Blastomycosis is a fungal disease that primarily affects dogs and humans and most commonly presents as pulmonary disease. Infection is typically acquired through inhalation of the environmental saprophytic mycelial form of the fungus (Ajellomyces dermatitidis) that, once within airways and at body temperature, propagates into yeast forms and establishes a primary infection in the lungs.3,8 After a primary infection is established, it can spread hematogenously throughout the body, with the eyes, skin, subcutis, lymph nodes, bones, and joints among the most common sites of dissemination.3,8 Although there have been rare reports of focal cutaneous infections from direct inoculation, and, within humans, rare reports of extrapulmonary infection, notably bone, without a known route of inoculation, in cases where the lungs are affected, the extrapulmonary lesions are generally regarded as sites of dissemination from the lungs rather than sites of primary inoculation.3, 5-9 In the dog of this case, along with the lungs, the connective tissue and skeletal muscle surrounding the right elbow were also affected. Although the dog initially presented for clinical signs referring to the elbow, based on the severity of pulmonary lesions, known pathogenesis of Blastomyces, and absence of a clinical history of penetrating trauma to the elbow, we favor primary pulmonary infection with dissemination to the joint. The lack of nuclear material / protoplasm in the yeasts in this case was interpreted as autolytic change.
Within the United States, Blastomyces dermatitidis is endemic to the Ohio River valleys, Mississippi, Missouri, and the mid-Atlantic states and is considered highly endemic along waterways in North Central Wisconsin, specifically near Eagle River.1,3 Defining the environmental niche of Blastomyces has been challenging due to difficulty isolating the organism from soil; however, a study of the endemic area of North Central Wisconsin revealed close proximity to waterways to be a risk factor for blastomycosis, which is part of what makes this region ideal for growth of the organism.1,11 Other environmental conditions that have been demonstrated to support its growth include acidic sandy soil, animal waste products, and wood by-products.3,8
Immunologic response to the Blastomyces infection is primarily mediated though a T-helper-1 (TH-1) immune response, which targets the primary virulence factor for Blastomyces, Blastomyces adhesion 1 (BAD-1) antigen. BAD-1 antigen has been demonstrated to promote cellular adhesion and invasion of mononuclear phagocytes and non-phagocytic cells and evades immune defenses by increasing production of transforming growth factor β and suppressing the production of tumor necrosis factor-α.3,11
Clinical signs of infection include cough, dyspnea, anorexia, weight loss, uveitis, and skin ulceration with drainage. Infection typically presents with purulent to pyogranulomatous lesions, with the most consistent finding being generalized coalescing, grey-white granulomatous nodules in the lungs.3,8 Histologically, infection is characterized by the presence of coalescing granulomas which surround round yeasts that measure 5-30 μm in diameter, have 1-μm thick double-contoured refractile walls, and central granular protoplasm. Yeasts occasionally exhibit broad-based budding. Infrequently, filamentous and pseudohyphal forms and conidia are found within tissues. Histologic diagnosis can be aided by the use of special stains, including histochemical staining by Gomori's methenamine silver (GMS) and periodic acid-Schiff (PAS) reaction.3,8
Contributing Institution:
University of Wisconsin
School of Veterinary Medicine
Department of Pathobiological Sciences
https://www.vetmed.wisc.edu/departments/pathobiological-sciences/
JPC
Diagnosis:
Lung: Pneumonia, interstitial, granulomatous, diffuse, severe, with intrahistiocytic and extracellular yeasts, etiology consistent with Blastomyces dermatitidis.
JPC Comment:
Although the pulmonary form is the most common manifestation of blastomycosis, this entity was first described in humans with cutaneous lesions. In 1894, Thomas Casper Gilchrist described ?peculiar parasitic bodies? he initially believed to be protozoans in sections of cutaneous tissue submitted from a patient in Philadelphia. However, he later realized the organism was a fungus after its isolation and named the agent Blastomyces dermatitidis; blastomycosis was subsequently known for some time as Gilchrist's disease.2,4
In 1898 Gilchrist published a report detailing a second case in a human patient, including detailed characteristics of the organism's characteristic budding and also noting yeasts were often enclosed within giants cells ??in various stages of germination?. Gilchrist then injected a suspension of the cultured Blastomyces dermatitidis into the jugular vein of a dog, which over the next two months became emaciated and had a nasal discharge. Both lungs ?presented a striking picture? upon opening the thoracic cavity, with numerous pea sized or larger, discrete, firm, light yellow nodules that upon histologic examination contained the characteristic yeast. The fungus was then cultured from the samples, essentially fulfilling Koch's postulates. Furthermore, Gilchrist intravenously inoculated two additional dogs, a sheep, and a horse with suspensions composed of lung nodules containing the organism. Pulmonary lesions containing the B. dermatitidis were identified in both dogs, the sheep, and the horse.4
Blastomycosis was initially thought to be restricted to North America and caused by a single species, Blastomyces dermatitidis. However, in 1952 a case was reported in Tunisia, with additional cases since reported in Africa and less commonly in India, the Middle East, and occasionally in Europe. In addition to B. dermatitidis, additional species have since been added to the genus, including B. gilchristii, B. percursus, and B. silverae. In addition, both Emmonsia parva and E. helica were reclassified as B. parva and B. helicus following a taxonomic revision in 2017 based on DNA sequence analysis. Interestingly, human cases of blastomycosis in Africa typically present as cutaneous lesions or osteomyelitis compared to pulmonary disease in North America. A recent retrospective study evaluating 20 human cases of blastomycosis in South Africa between 1967 and 2014 that had been attributed to B. dermatitidis were found to actually B. percursus and B. emzantsi based in ITS sequences. In addition, BAD-1, the important adhesion-promoting protein and virulence factor associated with B. dermatitidis could not be identified in these strains. Although BAD-1 has clearly been demonstrated to be an important virulence factor in North American strains, the significance of its absence in these African strains is unclear. However, it is possible the difference in virulence genes associated with the different strains may contribute to the variations of clinical manifestations between North American and African blastomycosis.10
Clinically, most canine patients present with normal complete blood count although mild anemia and mild neutrophila +/- mild left shift may be present. Hypoproteinemia is the most common abnormality noted on blood chemistry panels and mild hypercalcemia is present in approximately 10% of cases; most chemistry panels are normal. Radiographs reveal an interstitial pattern in approximately 70% of canine cases although nodular, bronchointerstital, and alveolar patterns have also been described. Osteolytic lesions may also be identified radiologically, particularly those affecting the long bones of the distal limbs, with the forelimbs affected more often than the pelvic limbs. An antigen enzyme immunoassay (EIA) for galactomannan, a sugar present in the cell wall of B. dermatitidis, can detect the substance in both urine and serum samples. However, galactomannan is only useful for determining the presence for a fungal infection in general as the test shows cross reactivity for blastomycosis, histoplasmosis, coccidiomycosis, penicilliosis, and paracoccidioidomycosis in humans and therefore most likely canines as well. Serology for antibodies is generally considered to be insensitive and non-specific, with one study reporting only 50% of dogs with confirmed blastomycosis to be positive. Agar-gel immunodiffusion (AGID) is the most commonly utilized serologic test although additional types include complement fixation, enzyme linked immunosorbent assay (ELISA), counterimmunoelectrophoresis, and agar-gel precipitation.12
Similar to as noted in WSC21-22 Conf. 14 case 2 with coccidiomycosis, the microbiology lab should be informed when samples are submitted for culture that are consistent with blastomycosis. This dimorphic fungus presents a serious risk to laboratory personnel as they may be exposed to the infective mycelial form if plates are mishandled.12
Conference participants discussed the variable patterns of pneumonia, including bronchopneumonia, interstitial, broncho-interstitial, and embolic. Bronchopneumonia is characterized by exudative lesions that originate at the bronchiolar-alveolar junction due causative agents entering via the airborne route and typically has a cranioventral distribution. Depending on individual and institutional preference, interstitial pneumonia may be used to describe a broad range of diseases that damage the interstitium (alveolar or interlobular septa) or be restricted to simply indicate increased numbers of leukocytes within alveolar septa. Diffuse alveolar damage is the most commonly recognized form of interstitial lung disease. Interstitial pneumonia typically has a diffuse distribution due to the causative agent being hematogenously distributed, such as in cases of endotoxemia and 3-metylindole toxicity in cattle. Bronchointerstitial pneumonia is also associated with two forms of use. In most cases, bronchointerstitial pneumonia indicates the presence of both bronchiolar necrosis and diffuse alveolar damage. However, the term has also been used to describe lesions in which airways are encircled by mononuclear cells that also infiltrate the alveolar septa. Embolic pneumonia occurs as the result of hematogenous distribution of infectious agents or inflammatory processes within the lung, such as with disseminated aspergillosis.3
The moderator also discussed additional infectious causes of granulomatous interstitial pneumonia in veterinary species, including other fungal agents such as Coccidioides, Histoplasma, and Cryptococcus, as well as bacterial agents such as Mycobacterium, Rhodococcus, Actinobacillus, Actinomyces, and Nocardia. Additional viral etiologies to be considered in canines with interstitial pneumonia include canine influenza, canine adenovirus-2, and canine distemper virus.
References:
1. Baumgardner DJ, Turkal NW, Paretsky DP. Blastomycosis in dogs: A fifteen-year survey in a very highly endemic area near Eagle River, Wisconsin, USA. Wilderness & Environmental Medicine 1996;7:1-8.
2. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-vii.
3. Caswell JL, Williams KJ. Respiratory System In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. St. Louis, Missouri: Elsevier, 2016;506-520, 580-581.
4. Gilchrist TC, Stokes WR. A CASE OF PSEUDO-LUPUS VULGARIS CAUSED BY A BLASTOMYCES. J Exp Med. 1898;3(1):53-78.
5. Graham WR, Callaway JL. Primary inoculation blastomycosis in a veterinarian. Journal of the American Academy of Dermatology 1982;7:785-786.
6. Harris JR, Blaney DD, Lindsley MD, et al. Blastomycosis in Man after Kinkajou Bite. Emerging Infectious Disease Journal 2011;17:268.
7. Jain R, Singh K, Lamzabi I, et al. Blastomycosis of Bone: A Clinicopathologic Study. American Journal of Clinical Pathology 2014;142:609-616.
8. Legendre AM. Blastomycosis In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Saunders, 2012;606-614
9. Lester RS, DeKoven JG, Kane J, et al. Novel cases of blastomycosis acquired in Toronto, Ontario. Canadian Medical Association Journal 2000;163:1309.
10. Maphanga TG, Birkhead M, Muñoz JF, et al. Human Blastomycosis in South Africa Caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967 to 2014. J Clin Microbiol. 2020;58(3):e01661-19. Published 2020 Feb 24.
11. McKinnell JA, Pappas PG. Blastomycosis: New Insights into Diagnosis, Prevention, and Treatment. Clinics in Chest Medicine 2009;30:227-239.
12. Taboada J, Grooters AM. Histoplasmosis, blastomycosis, sporotrichosis, candidiasis, pythiosis. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. Vol 1. 7th ed. St. Louis, MO: Saunders Elsevier; 2010: 997-998.