Wednesday Slide Conference, Conference 21, Case 4
Signalment:
9-week-old, female, human-peripheral blood mononuclear cell (huPBMC)-engrafted-NOD scid gamma (NSG) mouse (Mus musculus)
History:
Female, human peripheral blood mononuclear cell (hPBMC)-engrafted NOD scid gamma (NSG) mouse purchased from an approved vendor, was submitted from an experimental cohort of mice experiencing similar clinical course of disease. One week after arrival, this cohort underwent experimental injection of MDA-MB-231 human breast cancer cells into the subcutis of the left flank (tumor xenograft model). In the 2 weeks following injection, the cohort demonstrated significant weight loss (3-5g), poor tumor engraftment, and unexpectedly high mortality. This mouse was euthanized and submitted for post-mortem examination as a representative animal.
Gross Pathology:
The mouse weighed 16.15g and was in thin body condition (1.5/5 BCS); expected body weight for a female 9-week-old NSG mouse is 23.3 +/- 1.6g. There was diffuse pallor of the tail, ears and mucous membranes, and marked depletion of subcutaneous and visceral adipose. Scant white fascia-like tissue was noted at the tumor injection site, without evident tumor growth. The liver weighed 0.99 g (6.1% BW), was tan, and had rounded edges and a subtly irregular surface.
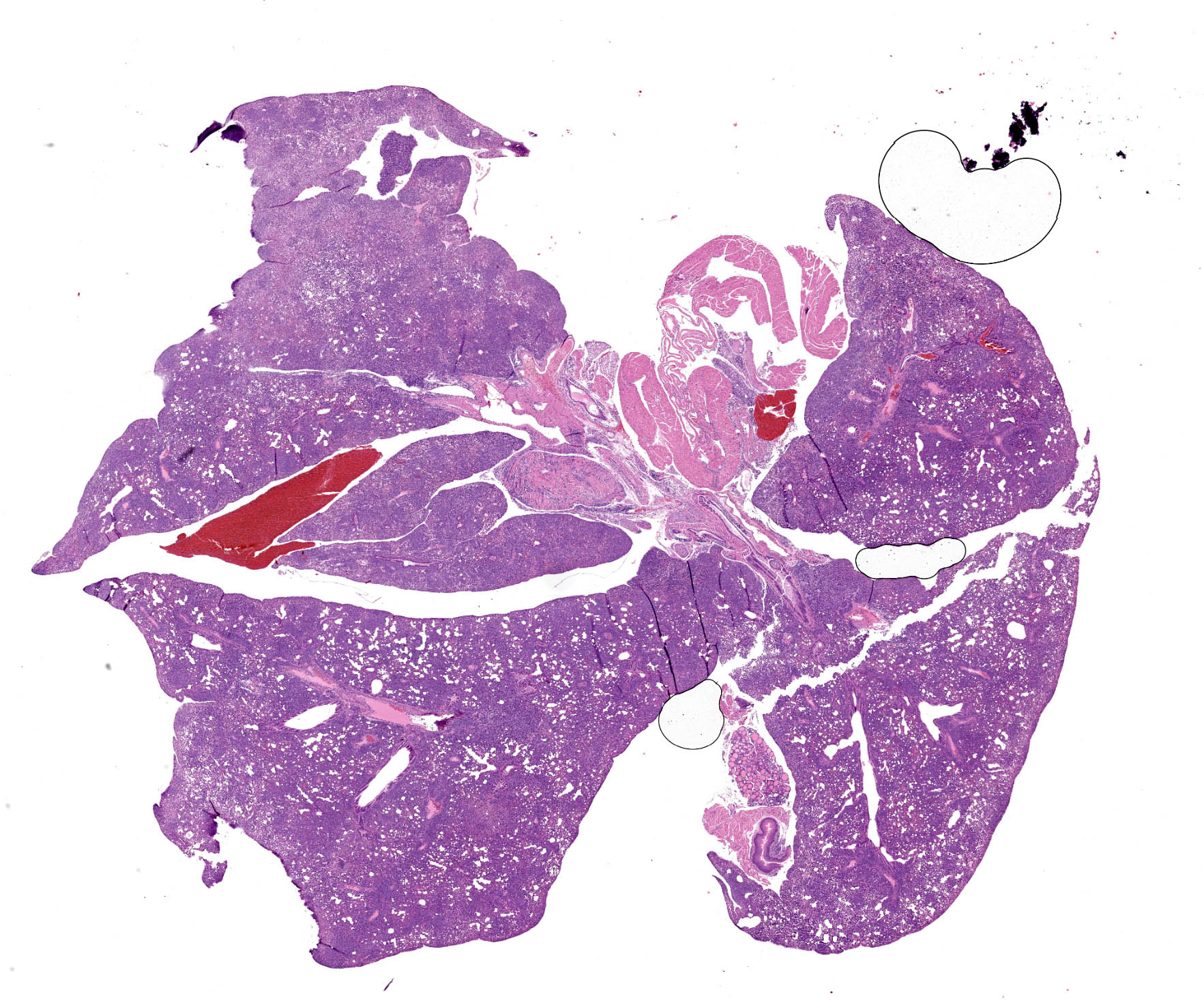
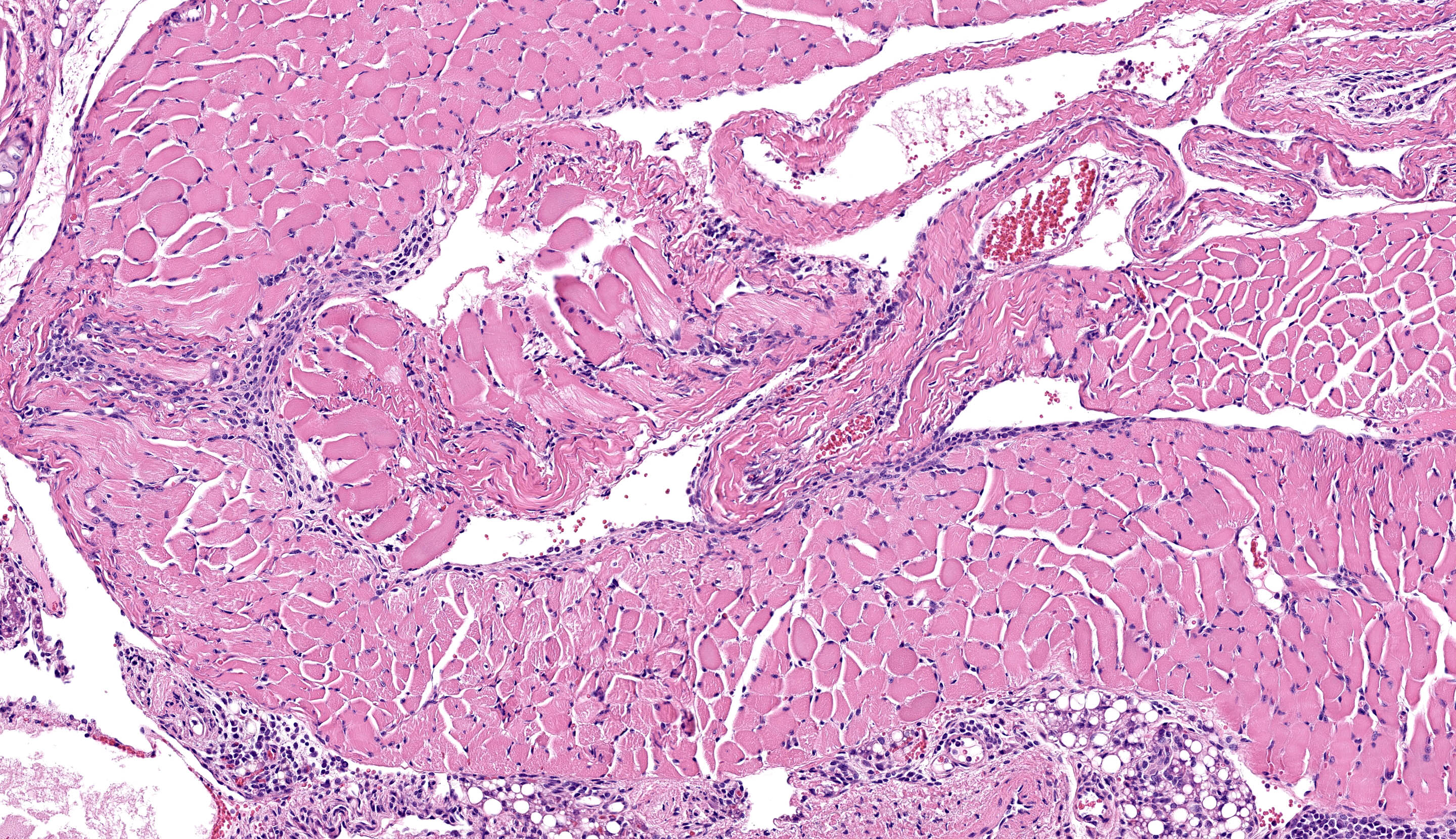
Microscopic Description:
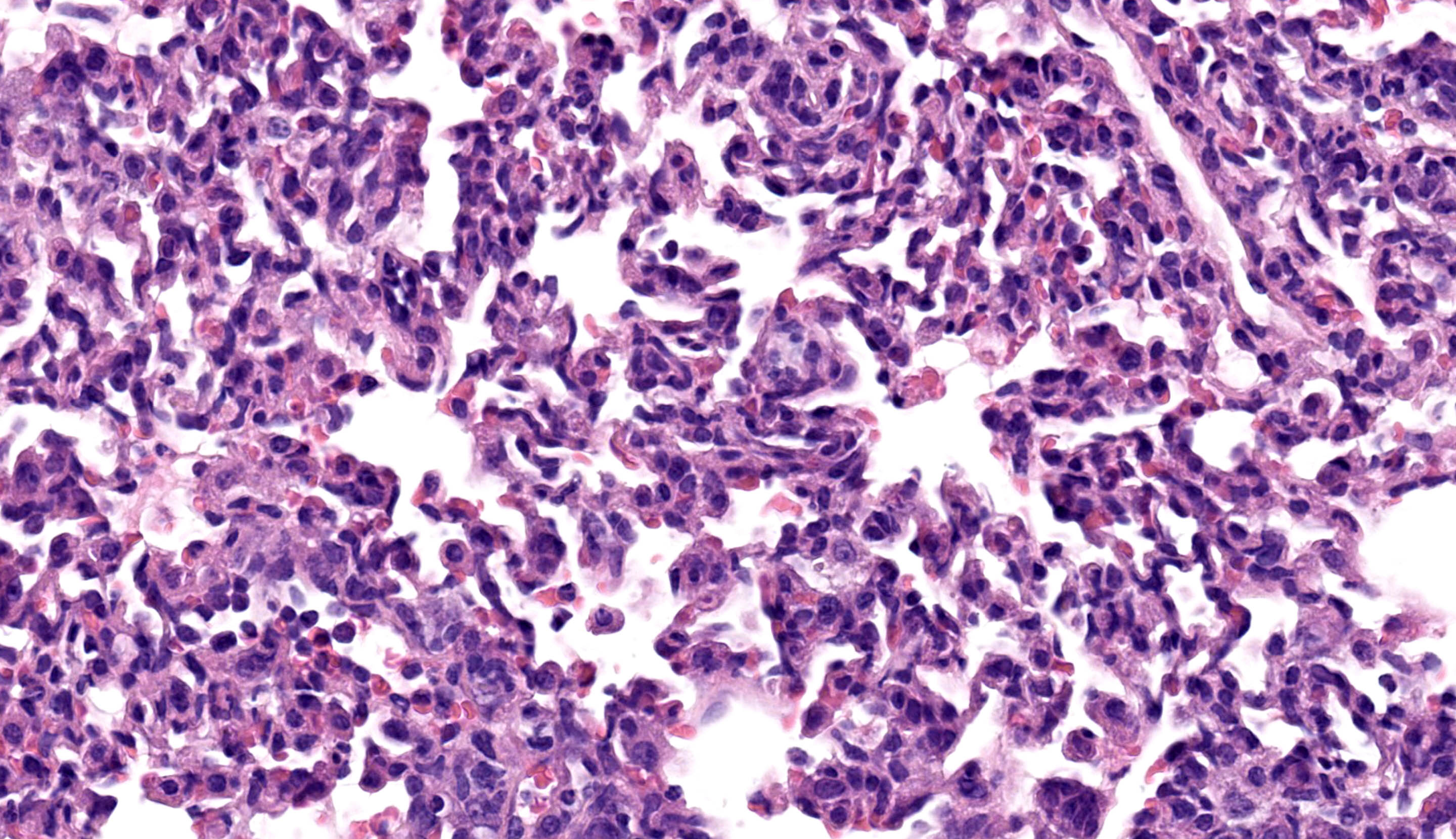
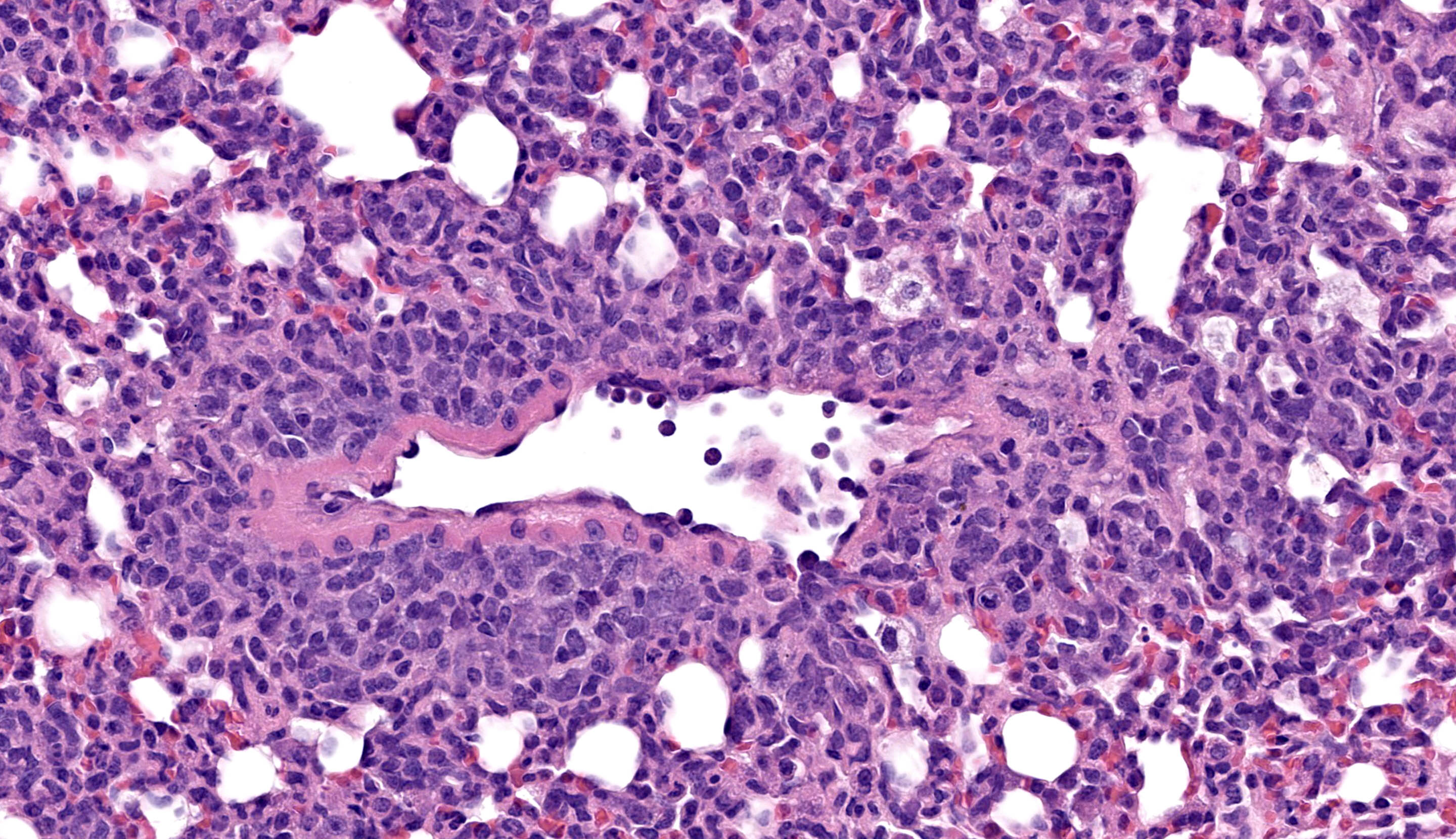
All lung lobes demonstrate multifocal to coalescing consolidation and atelectasis with interstitial and alveolar septal thickening by mononuclear inflammatory cells and plump spindle cells (fibroplasia). Alveoli are multifocally lined by a continuous layer of plump, cuboidal to polygonal, epithelial cells (type II pneumocyte hyperplasia). There are dense perivascular and peribronchiolar lymphocytic cuffs up to 15 cells thick that contain low to moderate numbers of mitotic figures and apoptotic bodies. Lymphocyte nuclei are subjectively slightly larger than expected (in mice).
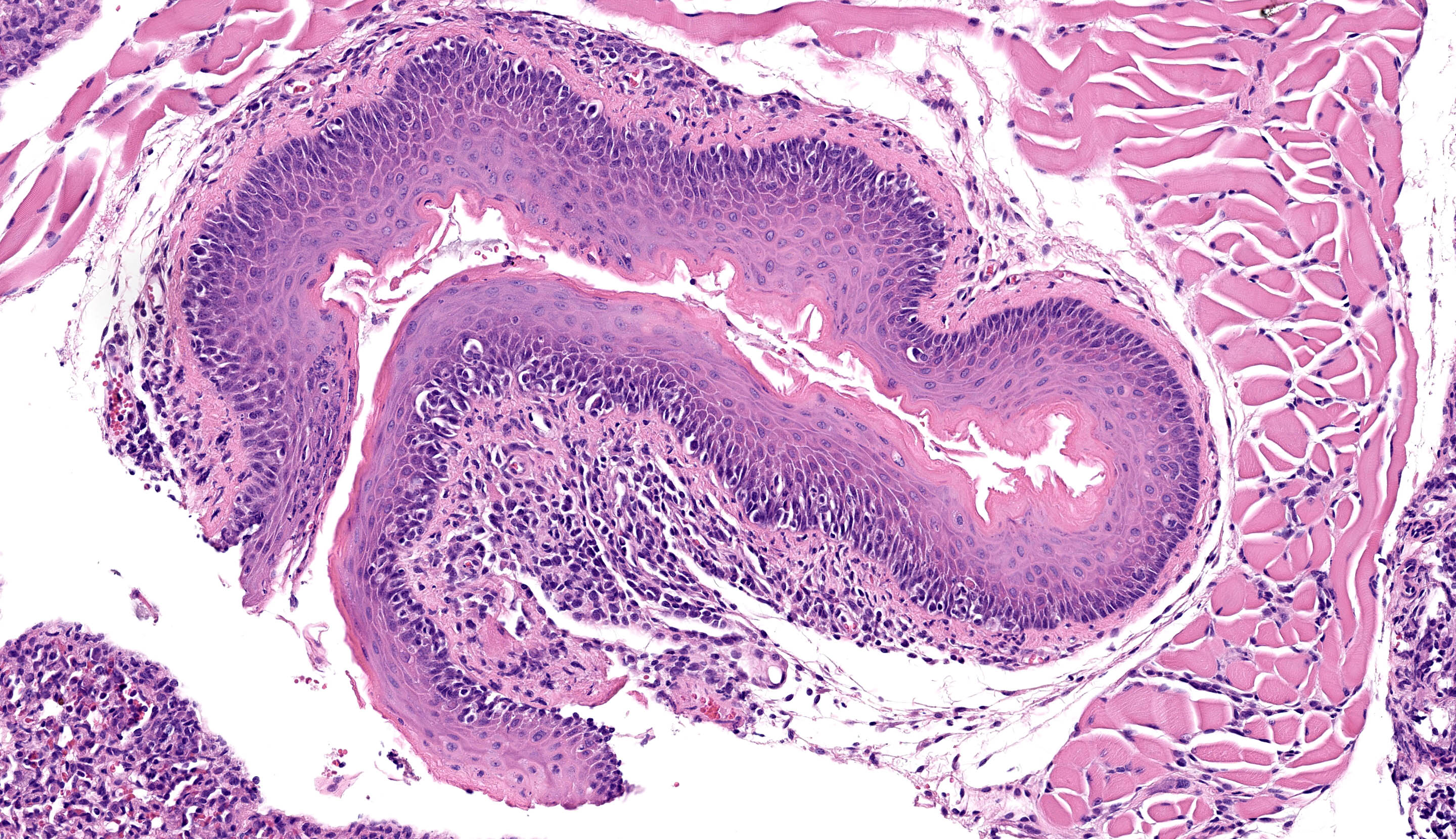
Esophageal mucosa has multifocal vacuolar degeneration and scattered apoptosis involving basal epithelial cells, and an underlying cell-rich band of lymphocytic inflammation with fewer macrophages and scattered neutrophils along the basement membrane interface. Lymphocytic and neutrophilic inflammation extends transmurally, with severe mononuclear infiltration and aggregation in the tunica muscularis. Associated changes in skeletal muscle fibers include: 1) rounded myofibers with pale sarcoplasm lacking cross-striations and internalization of nuclei (degeneration), 2) shrunken and rounded myofibers with small hyperchromatic nuclei (atrophy), or 3) effacement and replacement of myofibers by lymphocytic inflammation (myofiber loss).
Immunohistochemistry:
Immunohistochemistry confirmed that perivascular and peribronchiolar cuffs in the lung and interface inflammation in the esophagus are primarily CD3+ cells (consistent with T lymphocytes), with low to moderate numbers of Iba-1+ leukocytes (interpreted as macrophages).
Contributor’s Morphologic Diagnosis:
Lung:
Inflammation, perivascular to peribronchiolar to interstitial, lymphoproliferative and histiocytic, chronic and active, severe, with interstitial lung injury, chronic atelectasis, and type II pneumocyte hyperplasia
Esophagus:
Mucosa, basal epithelial cell vacuolization and apoptosis Esophagitis, ‘interface’, lymphocytic with fewer neutrophils and macrophages, multifocal to coalescing, severe, with marked submucosal and muscularis lymphocytic infiltration, and myofiber degeneration, atrophy and loss.
Condition:
Xenogeneic Graft versus Host Disease (GvHD)
Contributor’s Comment:
Dense multiorgan infiltration by large lymphocytes with associated epithelial degeneration/apoptosis and a 1) a lichenoid interface pattern in skin, mucous membranes and esophagus, 2) a perivascular distribution in lung, salivary gland, and pancreas, and 3) a portal distribution in the liver are consistent with Graft-versus-Host Disease (GvHD).10,11,14
GvHD is a life-threatening, systemic inflammatory condition mediated by alloreactive transplanted donor lymphocytes that recognize antigenic disparities between donor (graft) and recipient (host) tissues leading to a cell-mediated adaptive immune response. The principle (but not exclusive) antigens driving GvHD are differences in Major Histocompatibility Complex (MHC) class I and II expression between donor and recipient tissues, as these molecules can be highly polymorphic between individuals.8 MHC class I molecules are expressed on the cell surface of all nucleated cells, whereas MHC class II expression is largely limited to antigen presenting cells such as macrophages, dendritic cells, and B lymphocytes. A major component of T cell maturation in the thymus is the process of central tolerance where immature, self-reactive T cells are removed to prevent auto-immunity. However, in the setting of hematopoietic stem cell transplantation, the transferred immunocompetent donor T cells recognize host alloantigens as foreign, resulting in immune activation and targeting of host tissue.3,8 The primary effector cells are donor CD8+ T lymphocytes and Natural Killer cells that recognize host MHC I molecules and induce direct cytotoxicity in these cells. Alloreactive CD4+ T cells also contribute via recognition of MHC class II molecule differences and secretion of pro-inflammatory cytokines that induce further immune activation and tissue damage.3,8
NSG mice are an extremely immunodeficient mouse model that lack natural killer cells, B lymphocytes and T lymphocytes.5,12 Due to the lack of a functional adaptive immune system, these mice can be engrafted with human-derived tissues including hematopoietic stem cells, cell culture lines, and patient-derived xenografts.
Human peripheral blood mononuclear cell (hPBMC) NSG mice have become an important model in the study of GvHD, which is a major clinical problem in human hematopoietic stem cell recipients.4,12 hPBMC NSG mice are considered an improved model of xenogenic GvHD compared to NOD/SCID and RAG2null / IL2rγnull recipients due to their earlier onset of symptoms, more pronounced weight loss, and earlier mortality.4 Consequently, GvHD development is expected in hPBMC NSG, resulting in a shortened lifespan of approximately 3-4 months. The disease course in this case was more rapid than experienced previously by the submitting lab.
Notably, human T cells are able to recognize murine xeno-antigens presented by murine MHC class I and II molecules, and onset of GvHD is dependent on host expression of MHC.2,7 hPBMC NSG MHC I/II Double Knockout mouse models demonstrate delayed onset of xenogeneic-GvHD relative to hPBMC NSG mice, and thereby may be more suitable for immuno-oncology studies and/or long-term studies requiring human T cell engraftment.1,9
The two major subdivisions of GvHD are acute versus chronic GvHD, which are defined by their clinicopathologic features not solely the time after transplantation.3,13 Acute GvHD typically has rapid onset and is characterized by multiorgan lymphocytic, histiocytic, and neutrophilic inflammation with associated tissue destruction and epithelial apoptosis; the most commonly affected organs include the skin, gastrointestinal tract, and liver. In contrast, the hallmark of chronic GvHD is fibrotic/sclerotic lesions with variable leukocyte infiltration and can be observed in almost any organ.13 While chronic GvHD classically develops >100 days post-transplantation in patients that have previously developed acute GvHD, it can also develop de novo or simultaneously with acute GvHD.3,6,13 An abbreviated summary of the histologic criteria for acute and chronic GvHD and a comparison to features supportive of a diagnosis of xenogeneic GvHD in this case are included in Table 1.
Onset and progression of GvHD in this ‘humanized’ mouse was likely further accelerated and exacerbated by the additional injection of non-matched, human-derived tumor cells. Failure of tumor engraftment in these mice is also consistent with a hu-PBMC-derived immune response against the tumor.9 Given that the entire cohort of mice appeared affected, it is also possible these hPBMCs were unusually reactive resulting in accelerated onset of GvHD.
Expected lung changes in GvHD are characterized by intrabronchiolar T cells, apoptosis, and perivenulitis, which can progress acutely to respiratory distress and death, or to constrictive bronchiolitis obliterans (CBO) in chronic GvHD.11,14 Fibrosis was not prominent in these lung sections, which is attributed to the relatively short course of disease. Lung changes are more extensive than in similar cases here, and may suggest a role for opportunistic infection as a contributor to mortality in this cohort (e.g., Chlamydia spp. infection). Mycoplasma pulmonis and Filobacterium rodentium are excluded from rodent areas at this site. As bacteria were not identified with Gram stain and the clinical concern resolved after that cohort, further diagnostics were not pursued.
Contributing Institution:
Johns Hopkins University, School of Medicine
Department of Molecular and Comparative Pathobiology
https://mcp.bs.jhmi.edu/
JPC Diagnosis:
- Lung: Pneumonia, interstitial and lymphohistiocytic, subacute, diffuse, marked, with perivascular and peribronchiolar lymphoid proliferation.
- Esophagus: Esophagitis, interface and lymphocytic, subacute, multifocal, moderate.
- Skeletal muscle: Myositis, lymphocytic, subacute, multifocal, moderate.
- Thyroid gland: Thyroiditis, lymphocytic, subacute, multifocal, mild.
- Adipose tissue: Steatitis, lymphocytic, subacute, multifocal, mild.
JPC Comment:
The final case of this conference provided a healthy challenge for participants, with the knowledge of hematopoietic stem cell involvement in this humanized mouse being essential for understanding the underlying pathogenesis that the contributor nicely summarizes. We opted for multiple morphologic diagnoses to capture the distribution of inflammatory cells, though the underlying changes were similar across the lung, esophagus, skeletal muscle, thyroid, and adjacent adipose tissue. We did note a very mild acidophilic macrophage pneumonia in this mouse, though this is a non-specific finding and likely follows from the marked inflammation of the lung.
The contributor IHC results help to describe the distribution of inflammatory cells in this case. With the assistance of Dr. Radaelli, we further characterized these cells with the aid of a mouse-specific CD86 (B7; marker of antigen presenting cell), CD4/CD8 (T-cell markers), and CD79 (B-cell). CD86-positive cells were widely distributed in tissue while B-cells were sparse in the lung with none in esophagus or muscle. CD4+ T-helper cells were present in moderate numbers in the lung, muscle, and esophagus while CD8+ cytotoxic T-cells were notable in the lung, esophagus, and the thyroid with the latter not being as obvious on the H&E section. One limitation is that we did not have a human macrophage marker available (e.g. CD45) to fully demonstrate the xenogeneic attack inherent in this case however.
Finally, Dr. Radaelli discussed humanized mice as well as several of the undesired consequences of this exploratory model during his pre-conference lecture which we abridge here.9 Humanized mice are interspecies chimeras that offer a potential avenue to explore tumor oncogenic signatures (biomarkers), test therapeutic interventions, and assess relative toxicity therein through a stable line that supports continued/stable tumor growth. Because these mice are severely immunosuppressed and bathed in a veritable shower of cytokines to enhance human hematopoietic stem cell line proliferation, there is also the possibility of myeloid cell hyperactivation,15 excessive lymphoproliferation, and even oncogenic activation of latent viruses (e.g. Epstein-Barr virus) in human tissue grafts.16 Opportunistic infection, as seen in Case 1 of this conference, is yet another consideration. While the potential benefits of this model are significant, pathologists also play an important role in advocating for the welfare of these mice and identifying issues or limitations of a given mouse type.17
References:
- Brehm MA, Kenney LL, Wiles MV, et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019;33(3):3137-3151.
- Ehx G, Somja J, Warnatz HJ, et al. Xenogeneic Graft-Versus-Host Disease in Humanized NSG and NSG-HLA-A2/HHD Mice. Front Immunol. 2018;9:1943.
- Ghimire S, Weber D, Mavin E, Wang XN, Dickinson AM, Holler E. Pathophysiology of GvHD and Other HSCT-Related Major Complications. Front Immunol. 2017;8:79.
- Ito R, Katano I, Kawai K, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87(11):1654-1658.
- The Jackson Laboratory. Strain 005557 NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. Accessed June 28, 2023. https://www.jax.org/strain/005557.
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1.
- King MA, Covassin L, Brehm MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157(1):104-118.
- Kumar V, Abbas AK, Aster JC. Diseases of the immune system. In: Robbins and Cotran Pathologic Basis of Disease, 10th ed. Philadelphia, PA, Elsevier Saunders; 2021.
- Radaelli E, Hermans E, Omodho L, et al. Spontaneous Post-Transplant Disorders in NOD.Cg- Prkdcscid Il2rgtm1Sug/JicTac (NOG) Mice Engrafted with Patient-Derived Metastatic Melanomas. PLoS One. 2015;10(5):e0124974.
- Salomao M, Dorritie K, Mapara MY, Sepulveda A. Histopathology of Graft-vs-Host Disease of Gastrointestinal Tract and Liver: An Update. Am J Clin Pathol. 2016;145(5):591-603.
- Shulman HM, Cardona DM, Greenson JK, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21(4):589-603.
- Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786-798.
- Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702-708.
- Xu L, Drachenberg C, Tavora F, Burke A. Histologic findings in lung biopsies in patients with suspected graft-versus-host disease. Hum Pathol. 2013;44(7):1233-1240.
- Tarrant JC, Binder ZA, Bugatti M,et al. Pathology of macrophage activation syndrome in humanized NSGS mice. Res Vet Sci. 2021 Jan;134:137-146.
- Tillman H, Vogel P, Rogers T, Akers W, Rehg JE. Spectrum of Posttransplant Lymphoproliferations in NSG Mice and Their Association With EBV Infection After Engraftment of Pediatric Solid Tumors. Vet Pathol. 2020 May;57(3):445-456.
- Willis E, Verrelle J, Banerjee E,et al. Humanization with CD34-positive hematopoietic stem cells in NOG-EXL mice results in improved long-term survival and less severe myeloid cell hyperactivation phenotype relative to NSG-SGM3 mice. Vet Pathol. 2024 Jul;61(4):664-674.